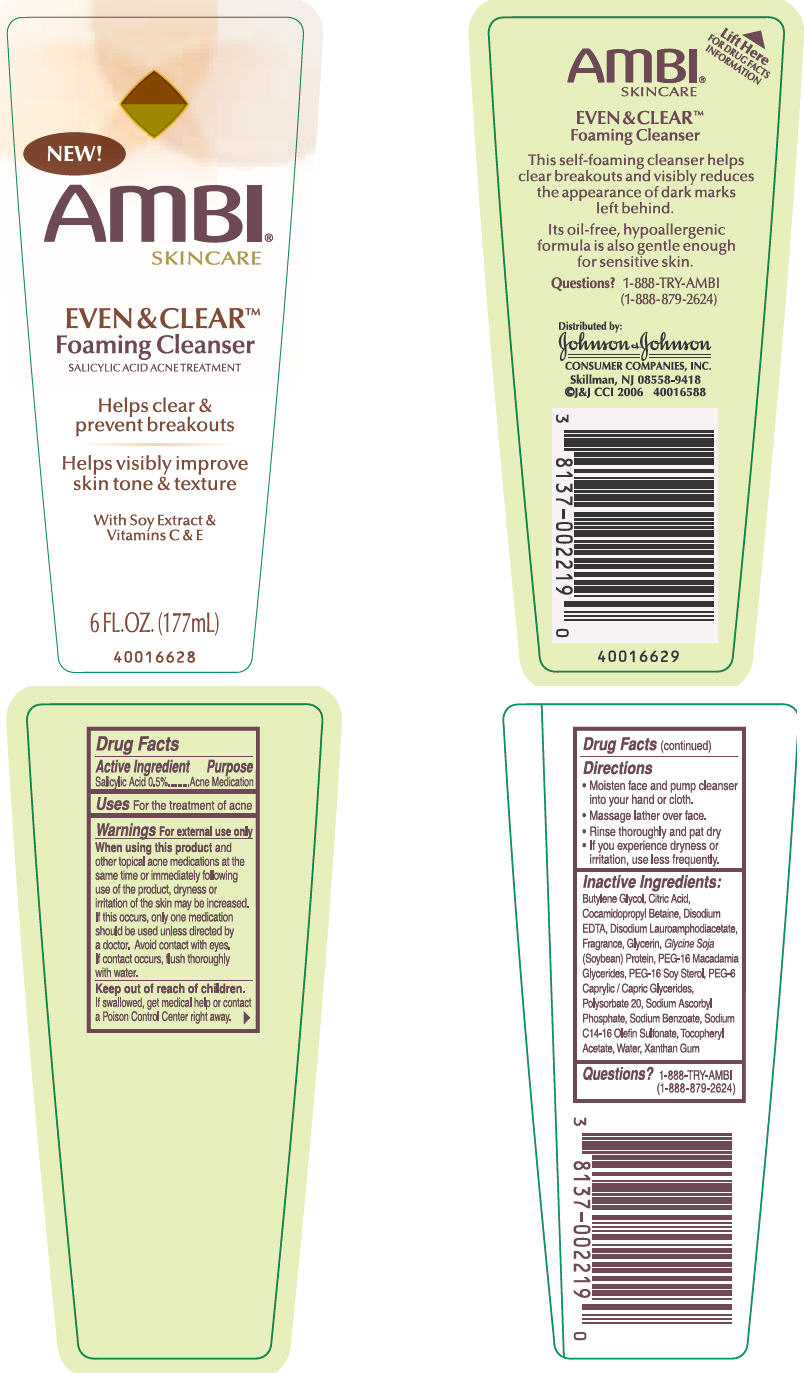
AMBI EVEN AND CLEAR FOAMING CLEANSER- salicylic acid liquid
Johnson & Johnson Consumer Products Company, Division of Johnson & Johnson Consumer Companies, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
When using this product and other topical acne medications at the same time or immediately following use of the product, dryness or irritation of the skin may be increased. If this occurs, only one medication should be used unless directed by a doctor. Avoid contact with eyes. If contact occurs, flush thoroughly with water.
Directions
- Moisten face and pump cleanser into your hand or cloth
- Massage lather over face.
- Rinse thoroughly and pat dry
- If you experience dryness or irritation, use less frequently.
Inactive ingredients
Butylene Glycol, Citric Acid, Cocamidopropyl Betaine, Disodium EDTA, Disodium Lauroamphodiacetate, Fragrance, Glycerin, Glycine Soja (Soybean) Protein, PEG-16 Macadamia Glycerides, PEG-16 Soy Sterol, PEG-6 Caprylic/Capric Glycerides, Polysorbate 20, Sodium Ascorbyl Phosphate, Sodium Benzoate, Sodium C14-16 Olefin Sulfonate, Tocopheryl Acetate, Water, Xanthan Gum
| AMBI EVEN AND CLEAR FOAMING CLEANSER
salicylic acid liquid |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Products Company, Division of Johnson & Johnson Consumer Companies, Inc. (830803164) |