ALAVERT ALLERGY SINUS D-12
-
loratadine and
pseudoephedrine sulfate tablet, film coated, extended release
Wyeth Consumer Healthcare
----------
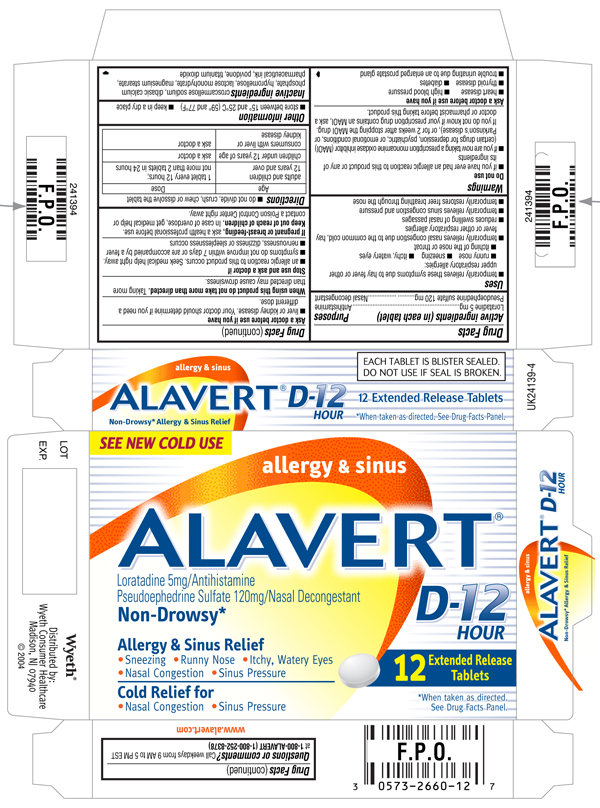
Alavert D-12 Hour Allergy and Sinus (Extended Release Tablets)(loratadine and pseudoephedrine sulfate)
DRUG FACTS
ACTIVE INGREDIENTS (IN EACH TABLET)
Pseudoephedrine sulfate 120 mg
PURPOSES
USES
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
WARNINGS
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed.
Taking more than directed may cause drowsiness.
Stop use and ask a doctor if
- an allergic reaction to this product occurs. Seek medical help right away.
- symptoms do not improve within 7 days or are accompanied by a fever
- nervousness, dizziness or sleeplessness occurs
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- do not divide, crush, chew or dissolve the tablet
| Age | Dose |
|---|---|
| adults and children 12 years and over | 1 tablet every 12 hours; not more than 2 tablets in 24 hours |
| children under 12 years of age | ask a doctor |
| consumers with liver or kidney disease | ask a doctor |
OTHER INFORMATION
- store between 15° and 25°C (59° and 77°F)
- keep in a dry place
INACTIVE INGREDIENTS
croscarmellose sodium, dibasic calcium phosphate, hypromellose, lactose monohydrate, magnesium stearate, pharmaceutical ink, povidone, titanium dioxide
QUESTIONS OR COMMENTS?
Call weekdays from 9 AM to 5 PM EST at 1-800-ALAVERT (1-800-252-8378)
PRODUCT PACKAGING
The product packaging shown below represents a sample of that currently in use. Additional packaging may also be available.
ALAVERT D-12 HOUR allergy & sinus
Pseudoephedrine Sulfate 120mg/Nasal Decongestant
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Nasal Congestion
- Sinus Pressure
- Nasal Congestion
- Sinus Pressure
*When taken as directed. See DRUG FACTS Panel.
EACH TABLET IS BLISTER SEALED. DO NOT USE IF SEAL IS BROKEN.
| ALAVERT ALLERGY SINUS D-12
loratadine, pseudoephedrine sulfate tablet, film coated, extended release |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA076050 | 01/30/2004 | |
| Labeler - Wyeth Consumer Healthcare (828831730) |