REA LO 40- urea cream
REA LO 40- urea lotion
Crown Laboratories
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Rea Lo 40
Rx Only
DESCRIPTION
Rea Lo 40® (Urea 40%) Cream and Lotion is a keratolytic emollient, which is a gentle, yet effective, tissue softener for skin. Each gram contains 400 mg Urea as the active ingredient and the following inactive ingredients: purified water, emulsifying wax, glycerin, isopropyl myristate, sorbitol, neopentyl glycol dicaprylate/dicaprate, tridecyl stearate, tridecyl trimellitate and dimethyl isosorbide.
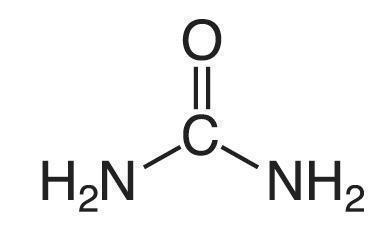
Urea is a diamide of carbonic acid with the following structure:
CLINICAL PHARMACOLOGY
Urea cream and lotion gently dissolves the intracellular matrix which results in loosening of the horny layer of the skin and shedding of scaly skin at regular intervals, thereby softening hyperkeratotic areas of the skin.
INDICATION AND USAGE
Rea Lo 40® (Urea 40%) Cream and Lotion is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, xerosis, ichthyosis, skin cracks and fissures, dermatitis, eczema, psoriasis, keratoses and calluses.
PRECAUTIONS
Urea cream and lotion should be used as directed by a physician and should not be used to treat conditions other than those for which it was prescribed. If redness or irritation occurs, discontinue use.
Pregnancy: Category C
Animal reproduction studies have not been conducted with urea cream or lotion. It is also not known whether urea cream or lotion can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. Urea cream or lotion should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether urea is excreted in human milk, therefore caution should be exercised when administering to a nursing mother.
ADVERSE REACTIONS
Transient stinging, burning, itching or irritation is possible and normally resolves upon discontinuing the medication.
DOSAGE AND ADMINISTRATION
Apply to affected skin twice per day, or as directed by your physician. Rub in until completely absorbed.
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF REACH OF CHILDREN
HOW SUPPLIED
The Rea Lo™ family of products includes various urea cream and lotion formulations.
The 40% urea products are available as:
Rea Lo 40® (Urea 40%) Cream supplied in:
1 oz (28.3 g) NDC 0316-07400-01
3 oz (85 g) NDC 0316-07400-03
7 oz (198.4 g) NDC 0316-07400-07
Rea Lo 40® (Urea 40%) Lotion supplied in:
8 fl oz (236.6 ml) NDC 0316-07401-08
Store at room temperature: 15˚C - 30˚C (59˚F - 86˚F).
Protect from freezing.
Manufactured by: Crown Laboratories, Inc. Johnson City, TN 37604
Rx Only
P19001.00
| REA LO 40
urea cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| REA LO 40
urea lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Crown Laboratories (079035945) |
| Registrant - Crown Laboratories (079035945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Crown Laboratories | 079035945 | manufacture(0316-7400) | |