ACTEMRA
-
tocilizumab injection, solution, concentrate
Genentech, Inc.
----------
|
||||||||||||||||||||||||
FULL PRESCRIBING INFORMATION
WARNING: RISK OF SERIOUS INFECTIONS
Patients treated with ACTEMRA are at increased risk for developing serious infections that may lead to hospitalization or death [see Warnings and Precautions (5.1), Adverse Reactions (6.1)]. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
If a serious infection develops, interrupt ACTEMRA until the infection is controlled.
Reported infections include:
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before ACTEMRA use and during therapy. Treatment for latent infection should be initiated prior to ACTEMRA use.
- Invasive fungal infections, including candidiasis, aspergillosis, and pneumocystis. Patients with invasive fungal infections may present with disseminated, rather than localized, disease.
- Bacterial, viral and other infections due to opportunistic pathogens.
The risks and benefits of treatment with ACTEMRA should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with ACTEMRA, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
ACTEMRA® (tocilizumab) is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies.
2 DOSAGE AND ADMINISTRATION
2.1 Rheumatoid Arthritis
ACTEMRA may be used as monotherapy or concomitantly with methotrexate or other DMARDs. The recommended dose of ACTEMRA for adult patients given once every 4 weeks as a 60-minute single intravenous drip infusion is:
| Recommended Adult Dosage Every 4 Weeks | |
|---|---|
| Patients who have had an inadequate response to one or more TNF antagonists | When used in combination with DMARDs or as monotherapy the recommended starting dose is 4 mg/kg followed by an increase to 8 mg/kg based on clinical response. |
- ACTEMRA has not been studied and its use should be avoided in combination with biological DMARDs such as TNF antagonists, IL-1R antagonists, anti-CD20 monoclonal antibodies and selective co-stimulation modulators because of the possibility of increased immunosuppression and increased risk of infection.
- It is recommended that ACTEMRA not be initiated in patients with an absolute neutrophil count (ANC) below 2000/mm3, platelet count below 100,000/mm3, or who have ALT or AST above 1.5 times the upper limit of normal (ULN).
- Reduction of dose from 8 mg/kg to 4 mg/kg is recommended for management of certain dose-related laboratory changes including elevated liver enzymes, neutropenia, and thrombocytopenia [see Dosage and Administration (2.3), Warnings and Precautions (5.3), and Adverse Reactions (6.1)].
- Doses exceeding 800 mg per infusion are not recommended [see Clinical Pharmacology (12.3)].
2.2 General Considerations for Administration
ACTEMRA for intravenous infusion should be diluted to 100 mL by a healthcare professional using aseptic technique as follows:
- From a 100 mL infusion bag or bottle, withdraw a volume of 0.9% Sodium Chloride Injection, USP, equal to the volume of the ACTEMRA solution required for the patient's dose.
- Slowly add ACTEMRA for intravenous infusion from each vial into the infusion bag or bottle. To mix the solution, gently invert the bag to avoid foaming.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If particulates and discolorations are noted, the product should not be used. Fully diluted ACTEMRA solutions are compatible with polypropylene, polyethylene and polyvinyl chloride infusion bags and polypropylene, polyethylene and glass infusion bottles.
- The fully diluted ACTEMRA solutions for infusion may be stored at 2°-8°C (36°-46°F) or room temperature for up to 24 hours and should be protected from light. ACTEMRA solutions do not contain preservatives; therefore, unused product remaining in the vials should not be used.
- Allow the fully diluted ACTEMRA solution to reach room temperature prior to infusion.
- The infusion should be administered over 60 minutes, and must be administered with an infusion set. Do not administer as an intravenous push or bolus.
- ACTEMRA should not be infused concomitantly in the same intravenous line with other drugs. No physical or biochemical compatibility studies have been conducted to evaluate the co-administration of ACTEMRA with other drugs.
2.3 Dosage Modifications
ACTEMRA treatment should be interrupted if a patient develops a serious infection until the infection is controlled.
| Liver Enzyme Abnormalities [see Warnings and Precautions (5.3)]: | |
|---|---|
| Lab Value | Recommendation |
| > 1 to 3x ULN | Dose modify concomitant DMARDs if appropriate |
| For persistent increases in this range, reduce ACTEMRA dose to 4 mg/kg or interrupt ACTEMRA until ALT/AST have normalized | |
| > 3 to 5x ULN | Interrupt ACTEMRA dosing until < 3x ULN and follow recommendations above for >1 to 3x ULN |
| (confirmed by repeat testing) | For persistent increases > 3x ULN, discontinue ACTEMRA |
| > 5x ULN | Discontinue ACTEMRA |
| Low Absolute Neutrophil Count (ANC) [see Warnings and Precautions (5.3)]: | |
|---|---|
| Lab Value (cells/mm3) | Recommendation |
| ANC > 1000 | Maintain dose |
| ANC 500 to 1000 | Interrupt ACTEMRA dosing When ANC > 1000 cells/mm3 resume ACTEMRA at 4 mg/kg and increase to 8 mg/kg as clinically appropriate |
| ANC < 500 | Discontinue ACTEMRA |
| Low Platelet Count [see Warnings and Precautions (5.3)]: | |
|---|---|
| Lab Value (cells/mm3) | Recommendation |
| 50,000 to 100,000 | Interrupt ACTEMRA dosing When platelet count is > 100,000 cells/mm3 resume ACTEMRA at 4 mg/kg and increase to 8 mg/kg as clinically appropriate |
| < 50,000 | Discontinue ACTEMRA |
3 DOSAGE FORMS AND STRENGTHS
Single-use vials of ACTEMRA (20 mg/mL):
- 80 mg/4 mL
- 200 mg/10 mL
- 400 mg/20 mL
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
Serious and sometimes fatal infections due to bacterial, mycobacterial, invasive fungal, viral, protozoal, or other opportunistic pathogens have been reported in patients receiving immunosuppressive agents including ACTEMRA for rheumatoid arthritis. The most common serious infections included pneumonia, urinary tract infection, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis and bacterial arthritis [see Adverse Reactions (6.1)]. Among opportunistic infections, tuberculosis, cryptococcus, aspergillosis, candidiasis, and pneumocystosis were reported with ACTEMRA. Other serious infections, not reported in clinical studies, may also occur (e.g., histoplasmosis, coccidioidomycosis, listeriosis). Patients have presented with disseminated rather than localized disease, and were often taking concomitant immunosuppressants such as methotrexate or corticosteroids which in addition to rheumatoid arthritis may predispose them to infections.
ACTEMRA should not be administered in patients with an active infection, including localized infections. The risks and benefits of treatment should be considered prior to initiating ACTEMRA in patients:
- with chronic or recurrent infection;
- who have been exposed to tuberculosis;
- with a history of serious or an opportunistic infection;
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses; or
- with underlying conditions that may predispose them to infection.
Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with ACTEMRA, as signs and symptoms of acute inflammation may be lessened due to suppression of the acute phase reactants [see Dosage and Administration (2.3), Adverse Reactions (6.1), and Patient Counseling Information (17.1)].
ACTEMRA should be interrupted if a patient develops a serious infection, an opportunistic infection, or sepsis. A patient who develops a new infection during treatment with ACTEMRA should undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, appropriate antimicrobial therapy should be initiated, and the patient should be closely monitored.
Tuberculosis
Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating ACTEMRA.
Anti-tuberculosis therapy should also be considered prior to initiation of ACTEMRA in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
Patients should be closely monitored for the development of signs and symptoms of tuberculosis including patients who tested negative for latent tuberculosis infection prior to initiating therapy.
It is recommended that patients be screened for latent tuberculosis infection prior to starting ACTEMRA. The incidence of tuberculosis in worldwide clinical development programs is 0.1%. Patients with latent tuberculosis should be treated with standard antimycobacterial therapy before initiating ACTEMRA.
Viral Reactivation
Viral reactivation has been reported with immunosuppressive biologic therapies and cases of herpes zoster exacerbation were observed in clinical studies with ACTEMRA. No cases of Hepatitis B reactivation were observed in the trials; however patients who screened positive for hepatitis were excluded.
5.2 Gastrointestinal Perforations
Events of gastrointestinal perforation have been reported in clinical trials, primarily as complications of diverticulitis. ACTEMRA should be used with caution in patients who may be at increased risk for gastrointestinal perforation. Patients presenting with new onset abdominal symptoms should be evaluated promptly for early identification of gastrointestinal perforation [see Adverse Reactions (6.1)].
5.3 Laboratory Parameters
Neutrophils
Treatment with ACTEMRA was associated with a higher incidence of neutropenia. Infections have been uncommonly reported in association with treatment-related neutropenia in long-term extension studies and postmarketing clinical experience.
- –
- It is not recommended to initiate ACTEMRA treatment in patients with a low neutrophil count i.e., absolute neutrophil count (ANC) <2000/mm3. In patients who develop an absolute neutrophil count <500/mm3 treatment is not recommended.
- –
- Neutrophils should be monitored every 4 to 8 weeks [see Clinical Pharmacology (12.2)]. For recommended modifications based on ANC results see Dosage and Administration (2.3).
Platelets
Treatment with ACTEMRA was associated with a reduction in platelet counts. Treatment-related reduction in platelets was not associated with serious bleeding events in clinical trials [see Adverse Reactions (6.1)].
- –
- It is not recommended to initiate ACTEMRA treatment in patients with a platelet count below 100,000/mm3. In patients who develop a platelet count <50,000/mm3 treatment is not recommended.
- –
- Platelets should be monitored every 4 to 8 weeks. For recommended modifications based on platelet counts see Dosage and Administration (2.3).
Liver Function Tests
Treatment with ACTEMRA was associated with a higher incidence of transaminase elevations. These elevations did not result in apparent permanent or clinically evident hepatic injury in clinical trials [see Adverse Reactions (6.1)]. Increased frequency and magnitude of these elevations was observed when potentially hepatotoxic drugs (e.g., MTX) were used in combination with ACTEMRA.
In one case, a patient who had received ACTEMRA 8 mg/kg monotherapy without elevations in transaminases experienced elevation in AST to above 10x ULN and elevation in ALT to above 16x ULN when MTX was initiated in combination with ACTEMRA. Transaminases normalized when both treatments were held, but elevations recurred when MTX and ACTEMRA were restarted at lower doses. Elevations resolved when MTX and ACTEMRA were discontinued.
- –
- It is not recommended to initiate ACTEMRA treatment in patients with elevated transaminases ALT or AST > 1.5x ULN. In patients who develop elevated ALT or AST > 5x ULN treatment is not recommended.
- –
- ALT and AST levels should be monitored every 4 to 8 weeks. When clinically indicated, other liver function tests such as bilirubin should be considered. For recommended modifications based on transaminases see Dosage and Administration (2.3).
Lipids
Treatment with ACTEMRA was associated with increases in lipid parameters such as total cholesterol, triglycerides, LDL cholesterol, and/or HDL cholesterol [see Adverse Reactions (6.1)].
- –
- Assessment of lipid parameters should be performed approximately 4 to 8 weeks following initiation of ACTEMRA therapy, then at approximately 6 month intervals.
- –
- Patients should be managed according to clinical guidelines [e.g., National Cholesterol Educational Program (NCEP)] for the management of hyperlipidemia.
5.4 Immunosuppression
The impact of treatment with ACTEMRA on the development of malignancies is not known but malignancies were observed in clinical studies [see Adverse Reactions (6.1)]. ACTEMRA is an immunosuppressant, and treatment with immunosuppressants may result in an increased risk of malignancies.
5.5 Hypersensitivity Reactions
Serious hypersensitivity reactions, including anaphylaxis, have been reported in association with infusion of ACTEMRA [see Adverse Reactions (6.1)]. Appropriate medical treatment should be available for immediate use in the event of an anaphylactic reaction during administration of ACTEMRA.
5.6 Demyelinating Disorders
The impact of treatment with ACTEMRA on demyelinating disorders is not known, but multiple sclerosis and chronic inflammatory demyelinating polyneuropathy were reported rarely in clinical studies. Patients should be closely monitored for signs and symptoms potentially indicative of demyelinating disorders. Prescribers should exercise caution in considering the use of ACTEMRA in patients with preexisting or recent onset demyelinating disorders.
5.7 Active Hepatic Disease and Hepatic Impairment
Treatment with ACTEMRA is not recommended in patients with active hepatic disease or hepatic impairment [see Adverse Reactions (6.1), Use in Specific Populations (8.6)].
5.8 Vaccinations
Live vaccines should not be given concurrently with ACTEMRA as clinical safety has not been established. No data are available on the secondary transmission of infection from persons receiving live vaccines to patients receiving ACTEMRA. No data are available on the effectiveness of vaccination in patients receiving ACTEMRA. Because IL-6 inhibition may interfere with the normal immune response to new antigens, patients should be brought up to date on all recommended vaccinations, except for live vaccines, prior to initiation of therapy with ACTEMRA.
6 ADVERSE REACTIONS
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not predict the rates observed in a broader patient population in clinical practice.
The ACTEMRA data described below includes 5 double-blind, controlled, multicenter studies. In these studies, patients received doses of ACTEMRA 8 mg/kg monotherapy (288 patients), ACTEMRA 8 mg/kg in combination with DMARDs (including methotrexate) (1582 patients), or ACTEMRA 4 mg/kg in combination with methotrexate (774 patients).
The all exposure population includes all patients in registration studies who received at least one dose of ACTEMRA. Of the 4009 patients in this population, 3577 received treatment for at least 6 months, 3296 for at least one year; 2806 received treatment for at least 2 years and 1222 for 3 years.
All patients in these studies had moderately to severely active rheumatoid arthritis. The study population had a mean age of 52 years, 82% were female and 74% were Caucasian.
6.1 Clinical Trials Experience
The most common serious adverse reactions were serious infections [see Warnings and Precautions (5.1)]. The most commonly reported adverse reactions in controlled studies up to 6 months (occurring in ≥ 5% of patients treated with ACTEMRA monotherapy or in combination with DMARDs) were upper respiratory tract infections, nasopharyngitis, headache, hypertension and increased ALT.
The proportion of patients who discontinued treatment due to any adverse reactions during the double-blind, placebo-controlled studies was 5% for patients taking ACTEMRA and 3% for placebo-treated patients. The most common adverse reactions that required discontinuation of ACTEMRA were increased hepatic transaminase values (per protocol requirement) and serious infections.
Overall Infections
In the 6-month, controlled clinical studies, the rate of infections in the ACTEMRA monotherapy group was 119 events per 100 patient-years and was similar in the methotrexate monotherapy group. The rate of infections in the 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD group was 133 and 127 events per 100 patient-years, respectively, compared to 112 events per 100 patient-years in the placebo plus DMARD group. The most commonly reported infections (5% to 8% of patients) were upper respiratory tract infections and nasopharyngitis.
The overall rate of infections with ACTEMRA in the all exposure population was 108 events per 100 patient-years.
Serious Infections
In the 6-month, controlled clinical studies, the rate of serious infections in the ACTEMRA monotherapy group was 3.6 per 100 patient-years compared to 1.5 per 100 patient-years in the methotrexate group. The rate of serious infections in the 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD group was 4.4 and 5.3 events per 100 patient-years, respectively, compared to 3.9 events per 100 patient-years in the placebo plus DMARD group.
In the all-exposure population, the overall rate of serious infections was 4.7 events per 100 patient-years. The most common serious infections included pneumonia, urinary tract infection, cellulitis, herpes zoster, gastroenteritis, diverticulitis, sepsis and bacterial arthritis. The overall rate of fatal serious infections was 0.13 per 100 patient-years. Cases of opportunistic infections have been reported [see Warnings and Precautions (5.1)].
Gastrointestinal Perforations
During the 6-month, controlled clinical trials, the overall rate of gastrointestinal perforation was 0.26 events per 100 patient-years with ACTEMRA therapy.
In the all-exposure population, the overall rate of gastrointestinal perforation was 0.28 events per 100 patient-years. Reports of gastrointestinal perforation were primarily reported as complications of diverticulitis including generalized purulent peritonitis, lower GI perforation, fistula and abscess. Most patients who developed gastrointestinal perforations were taking concomitant nonsteroidal anti-inflammatory medications (NSAIDs), corticosteroids, or methotrexate [see Warnings and Precautions (5.2)]. The relative contribution of these concomitant medications versus ACTEMRA to the development of GI perforations is not known.
Infusion Reactions
In the 6-month, controlled clinical studies, adverse events associated with the infusion (occurring during or within 24 hours of the start of infusion) were reported in 8% and 7% of patients in the 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD group, respectively, compared to 5% of patients in the placebo plus DMARD group. The most frequently reported event on the 4 mg/kg and 8 mg/kg dose during the infusion was hypertension (1% for both doses), while the most frequently reported event occurring within 24 hours of finishing an infusion were headache (1% for both doses) and skin reactions (1% for both doses), including rash, pruritus and urticaria. These events were not treatment limiting.
Clinically significant hypersensitivity reactions (e.g., anaphylactoid and anaphylactic reactions) associated with ACTEMRA and requiring treatment discontinuation were reported 0.1% (3/2644) in the 6-month, controlled trials and in 0.2% (9/4009) in the all-exposure population. These reactions were generally observed during the second to fourth infusion of ACTEMRA. Appropriate medical treatment should be available for immediate use in the event of a serious hypersensitivity reaction [see Warnings and Precautions (5.5)].
Laboratory Tests
Neutrophils
In the 6-month, controlled clinical studies, decreases in neutrophil counts below 1000/mm3 occurred in 1.8% and 3.4% of patients in the 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD group, respectively, compared to 0.1% of patients in the placebo plus DMARD group. Approximately half of the instances of ANC below 1000/mm3 occurred within 8 weeks of starting therapy. Decreases in neutrophil counts below 500/mm3 occurred in 0.4% and 0.3% of patients in the 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD, respectively, compared to 0.1% of patients in the placebo plus DMARD group. There was no clear relationship between decreases in neutrophils below 1000/mm3 and the occurrence of serious infections.
In the all-exposure population, the pattern and incidence of decreases in neutrophil counts remained consistent with what was seen in the 6-month controlled clinical studies [see Warnings and Precautions (5.3)].
Platelets
In the 6-month, controlled clinical studies, decreases in platelet counts below 100,000/mm3 occurred in 1.3% and 1.7% of patients on 4 mg/kg and 8 mg/kg ACTEMRA plus DMARD, respectively, compared to 0.5% of patients on placebo plus DMARD, without associated bleeding events.
In the all-exposure population, the pattern and incidence of decreases in platelet counts remained consistent with what was seen in the 6-month controlled clinical studies [see Warnings and Precautions (5.3)].
Liver Function Tests
Liver enzyme abnormalities are summarized in Table 1. In patients experiencing liver enzyme elevation, modification of treatment regimen, such as reduction in the dose of concomitant DMARD, interruption of ACTEMRA, or reduction in ACTEMRA dose, resulted in decrease or normalization of liver enzymes [see Dosage and Administration (2.3)]. These elevations were not associated with clinically relevant increases in direct bilirubin, nor were they associated with clinical evidence of hepatitis or hepatic insufficiency [see Warnings and Precautions (5.3)].
| ACTEMRA 8 mg/kg MONOTHERAPY | Methotrexate | ACTEMRA 4 mg/kg + DMARDs | ACTEMRA 8 mg/kg + DMARDs | Placebo + DMARDs | |
|---|---|---|---|---|---|
| N = 288 (%) | N = 284 (%) | N = 774 (%) | N = 1582 (%) | N = 1170 (%) |
|
| ULN = Upper Limit of Normal | |||||
|
|||||
| AST (U/L) | |||||
| > ULN to 3x ULN | 22 | 26 | 34 | 41 | 17 |
| > 3x ULN to 5x ULN | 0.3 | 2 | 1 | 2 | 0.3 |
| > 5x ULN | 0.7 | 0.4 | 0.1 | 0.2 | < 0.1 |
| ALT (U/L) | |||||
| > ULN to 3x ULN | 36 | 33 | 45 | 48 | 23 |
| > 3x ULN to 5x ULN | 1 | 4 | 5 | 5 | 1 |
| > 5x ULN | 0.7 | 1 | 1.3 | 1.5 | 0.3 |
Lipids
Elevations in lipid parameters (total cholesterol, LDL, HDL, triglycerides) were first assessed at 6 weeks following initiation of ACTEMRA in the controlled 6-month clinical trials. Increases were observed at this time point and remained stable thereafter. Increases in triglycerides to levels above 500 mg/dL were rarely observed. Changes in other lipid parameters from baseline to week 24 were evaluated and are summarized below:
- –
- Mean LDL increased by 13 mg/dL in the TCZ 4 mg/kg+DMARD arm, 20 mg/dL in the TCZ 8 mg/kg+DMARD, and 25 mg/dL in TCZ 8 mg/kg monotherapy.
- –
- Mean HDL increased by 3 mg/dL in the TCZ 4 mg/kg+DMARD arm, 5 mg/dL in the TCZ 8 mg/kg+DMARD, and 4 mg/dL in TCZ 8 mg/kg monotherapy.
- –
- Mean LDL/HDL ratio increased by an average of 0.14 in the TCZ 4 mg/kg+DMARD arm, 0.15 in the TCZ 8 mg/kg+DMARD, and 0.26 in TCZ 8 mg/kg monotherapy.
- –
- ApoB/ApoA1 ratios were essentially unchanged in ACTEMRA-treated patients.
Elevated lipids responded to lipid lowering agents.
Immunogenicity
In the 6-month, controlled clinical studies, a total of 2876 patients have been tested for anti-tocilizumab antibodies. Forty-six patients (2%) developed positive anti-tocilizumab antibodies, of whom 5 had an associated, medically significant, hypersensitivity reaction leading to withdrawal. Thirty patients (1%) developed neutralizing antibodies.
The data reflect the percentage of patients whose test results were positive for antibodies to tocilizumab in specific assays. The observed incidence of antibody positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to tocilizumab with the incidence of antibodies to other products may be misleading.
Malignancies
During the 6-month, controlled period of the studies, 15 malignancies were diagnosed in patients receiving ACTEMRA, compared to 8 malignancies in patients in the control groups. Exposure-adjusted incidence was similar in the ACTEMRA groups (1.32 events per 100 patient-years) and in the placebo plus DMARD group (1.37 events per 100 patient-years).
In the all-exposure population, the rate of malignancies remained consistent (1.10 events per 100 patient-years) with the rate observed in the 6-month, controlled period [see Warnings and Precautions (5.4)].
Other Adverse Reactions
Adverse reactions occurring in 2% or more of patients on 4 or 8 mg/kg ACTEMRA plus DMARD and at least 1% greater than that observed in patients on placebo plus DMARD are summarized in Table 2.
| 6 Month Phase 3 Controlled Study Population | |||||
|---|---|---|---|---|---|
| ACTEMRA 8 mg/kg MONOTHERAPY | Methotrexate | ACTEMRA 4 mg/kg + DMARDs | ACTEMRA 8 mg/kg + DMARDs | Placebo + DMARDs | |
| Preferred Term | N = 288 (%) | N = 284 (%) | N = 774 (%) | N = 1582 (%) | N = 1170 (%) |
| Upper Respiratory Tract Infection | 7 | 5 | 6 | 8 | 6 |
| Nasopharyngitis | 7 | 6 | 4 | 6 | 4 |
| Headache | 7 | 2 | 6 | 5 | 3 |
| Hypertension | 6 | 2 | 4 | 4 | 3 |
| ALT increased | 6 | 4 | 3 | 3 | 1 |
| Dizziness | 3 | 1 | 2 | 3 | 2 |
| Bronchitis | 3 | 2 | 4 | 3 | 3 |
| Rash | 2 | 1 | 4 | 3 | 1 |
| Mouth Ulceration | 2 | 2 | 1 | 2 | 1 |
| Abdominal Pain Upper | 2 | 2 | 3 | 3 | 2 |
| Gastritis | 1 | 2 | 1 | 2 | 1 |
| Transaminase increased | 1 | 5 | 2 | 2 | 1 |
7 DRUG INTERACTIONS
7.1 Other Drugs for Treatment of Rheumatoid Arthritis
Population pharmacokinetic analyses did not detect any effect of methotrexate, non-steroidal anti-inflammatory drugs or corticosteroids on tocilizumab clearance.
Concomitant administration of a single dose of 10 mg/kg ACTEMRA with 10-25 mg MTX once weekly had no clinically significant effect on MTX exposure.
ACTEMRA has not been studied in combination with biological DMARDs such as TNF antagonists [see Dosage and Administration (2.1)].
7.2 Interactions with CYP450 Substrates
Cytochrome P450s in the liver are down-regulated by infection and inflammation stimuli including cytokines such as IL-6. Inhibition of IL-6 signaling in RA patients treated with tocilizumab may restore CYP450 activities to higher levels than those in the absence of tocilizumab leading to increased metabolism of drugs that are CYP450 substrates. In vitro studies showed that tocilizumab has the potential to affect expression of multiple CYP enzymes including CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4. Its effects on CYP2C8 or transporters is unknown. In vivo studies with omeprazole, metabolized by CYP2C19 and CYP3A4, and simvastatin, metabolized by CYP3A4, showed up to a 28% and 57% decrease in exposure one week following a single dose of ACTEMRA, respectively. The effect of tocilizumab on CYP enzymes may be clinically relevant for CYP450 substrates with narrow therapeutic index, where the dose is individually adjusted. Upon initiation or discontinuation of ACTEMRA, in patients being treated with these types of medicinal products, therapeutic monitoring of effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) should be performed and the individual dose of the medicinal product adjusted as needed. Prescribers should exercise caution when ACTEMRA is coadministered with CYP3A4 substrate drugs where decrease in effectiveness is undesirable, e.g., oral contraceptives, lovastatin, atorvastatin, etc. The effect of tocilizumab on CYP450 enzyme activity may persist for several weeks after stopping therapy [see Clinical Pharmacology (12.3)].
7.3 Live Vaccines
Live vaccines should not be given concurrently with ACTEMRA [see Warnings and Precautions (5.8)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. ACTEMRA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
An embryo-fetal developmental toxicity study was performed in which pregnant cynomolgus monkeys were treated intravenously with tocilizumab (daily doses of 2, 10, or 50 mg/kg from gestation day 20-50) during organogenesis. Although there was no evidence for a teratogenic/dysmorphogenic effect at any dose, tocilizumab produced an increase in the incidence of abortion/embryo-fetal death at 10 mg/kg and 50 mg/kg doses (1.25 and 6.25 times the human dose of 8 mg/kg every 4 weeks based on a mg/kg comparison).
Nonteratogenic Effects. Testing of a murine analogue of tocilizumab in mice did not yield any evidence of harm to offspring during the pre- and postnatal development phase when dosed at 50 mg/kg intravenously with treatment every three days from implantation until day 21 after delivery (weaning). There was no evidence for any functional impairment of the development and behavior, learning ability, immune competence and fertility of the offspring.
Pregnancy Registry: To monitor the outcomes of pregnant women exposed to ACTEMRA, a pregnancy registry has been established. Physicians are encouraged to register patients and pregnant women are encouraged to register themselves by calling 1-877-311-8972.
8.3 Nursing Mothers
It is not known whether tocilizumab is excreted in human milk or absorbed systemically after ingestion. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from ACTEMRA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness of ACTEMRA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 2644 patients who received ACTEMRA in Studies I to V [see Clinical Studies (14)], a total of 435 rheumatoid arthritis patients were 65 years of age and older, including 50 patients 75 years and older. The frequency of serious infection among ACTEMRA treated subjects 65 years of age and older was higher than those under the age of 65. As there is a higher incidence in infections in the elderly population in general, caution should be used when treating the elderly.
8.6 Hepatic Impairment
The safety and efficacy of ACTEMRA have not been studied in patients with hepatic impairment, including patients with positive HBV and HCV serology [see Warnings and Precautions (5.7)].
8.7 Renal Impairment
No dose adjustment is required in patients with mild renal impairment. ACTEMRA has not been studied in patients with moderate to severe renal impairment [see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
No studies on the potential for ACTEMRA to cause dependence have been performed. However, there is no evidence from the available data that ACTEMRA treatment results in dependence.
10 OVERDOSAGE
There are limited data available on overdoses with ACTEMRA. One case of accidental overdose was reported in which a patient with multiple myeloma received a dose of 40 mg/kg. No adverse drug reactions were observed. No serious adverse drug reactions were observed in healthy volunteers who received single doses of up to 28 mg/kg, although all 5 patients at the highest dose of 28 mg/kg developed dose-limiting neutropenia.
In case of an overdose, it is recommended that the patient be monitored for signs and symptoms of adverse reactions. Patients who develop adverse reactions should receive appropriate symptomatic treatment.
11 DESCRIPTION
ACTEMRA (tocilizumab) is a recombinant humanized anti-human interleukin 6 (IL-6) receptor monoclonal antibody of the immunoglobulin IgG1κ (gamma 1, kappa) subclass with a typical H2L2 polypeptide structure. Each light chain and heavy chain consists of 214 and 448 amino acids, respectively. The four polypeptide chains are linked intra- and inter-molecularly by disulfide bonds. ACTEMRA has a molecular weight of approximately 148 kDa.
ACTEMRA is supplied as a sterile, preservative-free solution for intravenous (IV) infusion at a concentration of 20 mg/mL. ACTEMRA is a colorless to pale yellow liquid, with a pH of about 6.5. Single-use vials are available containing 80 mg/4 mL, 200 mg/10 mL, or 400 mg/20 mL of ACTEMRA. Injectable solutions of ACTEMRA are formulated in an aqueous solution containing disodium phosphate dodecahydrate and sodium dihydrogen phosphate dehydrate (as a 15 mmol/L phosphate buffer), polysorbate 80 (0.5 mg/mL), and sucrose (50 mg/mL).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tocilizumab binds specifically to both soluble and membrane-bound IL-6 receptors (sIL-6R and mIL-6R), and has been shown to inhibit IL-6-mediated signaling through these receptors. IL-6 is a pleiotropic pro-inflammatory cytokine produced by a variety of cell types including T- and B-cells, lymphocytes, monocytes and fibroblasts. IL-6 has been shown to be involved in diverse physiological processes such as T-cell activation, induction of immunoglobulin secretion, initiation of hepatic acute phase protein synthesis, and stimulation of hematopoietic precursor cell proliferation and differentiation. IL-6 is also produced by synovial and endothelial cells leading to local production of IL-6 in joints affected by inflammatory processes such as rheumatoid arthritis.
12.2 Pharmacodynamics
In clinical studies with the 4 mg/kg and 8 mg/kg doses of ACTEMRA, decreases in levels of C-reactive protein (CRP) to within normal ranges were seen as early as week 2. Changes in pharmacodynamic parameters were observed (i.e., decreases in rheumatoid factor, erythrocyte sedimentation rate, serum amyloid A and increases in hemoglobin) with both doses, however the greatest improvements were observed with 8 mg/kg ACTEMRA.
In healthy subjects administered ACTEMRA in doses from 2 to 28 mg/kg, absolute neutrophil counts decreased to the nadir 3 to 5 days following ACTEMRA administration. Thereafter, neutrophils recovered towards baseline in a dose dependent manner. Rheumatoid arthritis patients demonstrated a similar pattern of absolute neutrophil counts following ACTEMRA administration [see Warnings and Precautions (5.3)].
12.3 Pharmacokinetics
The pharmacokinetics characterized in healthy subjects and RA patients suggested that PK is similar between the two populations. The clearance (CL) of tocilizumab decreased with increased doses. At the 10 mg/kg single dose in RA patients, mean CL was 0.29 ± 0.10 mL/hr/kg and mean apparent terminal t1/2 was 151 ± 59 hours (6.3 days).
The pharmacokinetics of tocilizumab were determined using a population pharmacokinetic analysis of 1793 rheumatoid arthritis patients treated with ACTEMRA 4 and 8 mg/kg every 4 weeks for 24 weeks.
The pharmacokinetic parameters of tocilizumab did not change with time. A more than dose-proportional increase in area under the curve (AUC) and trough concentration (Cmin) was observed for doses of 4 and 8 mg/kg every 4 weeks. Maximum concentration (Cmax) increased dose-proportionally. At steady-state, predicted AUC and Cmin were 2.7 and 6.5-fold higher at 8 mg/kg as compared to 4 mg/kg, respectively.
For doses of ACTEMRA 4 mg/kg given every 4 weeks, the predicted mean (± SD) steady-state AUC, Cmin and Cmax of tocilizumab were 13000 ± 5800 mcg•h/mL, 1.49 ± 2.13 mcg/mL, and 88.3 ± 41.4 mcg/mL, respectively. The accumulation ratios for AUC and Cmax were 1.11 and 1.02, respectively. The accumulation ratio was higher for Cmin (1.96). Steady-state was reached following the first administration for Cmax and AUC, respectively, and after 16 weeks Cmin.
For doses of ACTEMRA 8 mg/kg given every 4 weeks, the predicted mean (± SD) steady-state AUC, Cmin and Cmax of tocilizumab were 35000 ± 15500 mcg•h/mL, 9.74 ± 10.5 mcg/mL, and 183 ± 85.6 mcg/mL, respectively. The accumulation ratios for AUC and Cmax were 1.22 and 1.06, respectively. The accumulation ratio was higher for Cmin (2.35). Steady-state was reached following the first administration and after 8 and 20 weeks for Cmax, AUC, and Cmin, respectively. Tocilizumab AUC, Cmin and Cmax increased with increase of body weight. At body weight ≥ 100 kg, the predicted mean (± SD) steady-state AUC, Cmin and Cmax of tocilizumab were 55500 ± 14100 mcg•h/mL, 19.0 ± 12.0 mcg/mL, and 269 ± 57 mcg/mL, respectively, which are higher than mean exposure values for the patient population. Therefore, ACTEMRA doses exceeding 800 mg per infusion are not recommended [see Dosage and Administration (2.1)].
Distribution
Following intravenous dosing, tocilizumab undergoes biphasic elimination from the circulation. In rheumatoid arthritis patients the central volume of distribution was 3.5 L and the peripheral volume of distribution was 2.9 L, resulting in a volume of distribution at steady state of 6.4 L.
Elimination
The total clearance of tocilizumab is concentration-dependent and is the sum of the linear clearance and the nonlinear clearance. The linear clearance was estimated to be 12.5 mL/h in the population pharmacokinetic analysis. The concentration-dependent nonlinear clearance plays a major role at low tocilizumab concentrations. Once the nonlinear clearance pathway is saturated, at higher tocilizumab concentrations, clearance is mainly determined by the linear clearance.
The t1/2 of tocilizumab is concentration-dependent. The concentration-dependent apparent t1/2 is up to 11 days for 4 mg/kg and up to 13 days for 8 mg/kg every 4 weeks at steady-state.
Pharmacokinetics in Special Populations
Population pharmacokinetic analyses in adult rheumatoid arthritis patients showed that age, gender and race did not affect the pharmacokinetics of tocilizumab. Linear clearance was found to increase with body size. The body weight-based dose (8 mg/kg) resulted in approximately 86% higher exposure in patients who are greater than 100 kg in comparison to patients who are less than 60 kg.
Hepatic Impairment
No formal study of the effect of hepatic impairment on the pharmacokinetics of tocilizumab was conducted.
Renal Impairment
No formal study of the effect of renal impairment on the pharmacokinetics of tocilizumab was conducted.
Most patients in the population pharmacokinetic analysis had normal renal function or mild renal impairment. Mild renal impairment (creatinine clearance < 80 mL/min and ≥ 50 mL/min based on Cockcroft-Gault) did not impact the pharmacokinetics of tocilizumab. No dose adjustment is required in patients with mild renal impairment.
Drug Interactions
In vitro data suggested that IL-6 reduced mRNA expression for several CYP450 isoenzymes including CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4, and this reduced expression was reversed by co-incubation with tocilizumab at clinically relevant concentrations. Accordingly, inhibition of IL-6 signaling in RA patients treated with tocilizumab may restore CYP450 activities to higher levels than those in the absence of tocilizumab leading to increased metabolism of drugs that are CYP450 substrates. Its effect on CYP2C8 or transporters (e.g., P-gp) is unknown. This is clinically relevant for CYP450 substrates with a narrow therapeutic index, where the dose is individually adjusted. Upon initiation of ACTEMRA, in patients being treated with these types of medicinal products, therapeutic monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) should be performed and the individual dose of the medicinal product adjusted as needed. Caution should be exercised when ACTEMRA is coadministered with drugs where decrease in effectiveness is undesirable, e.g., oral contraceptives (CYP3A4 substrates) [see Drug Interactions (7.2)].
Simvastatin
Simvastatin is a CYP3A4 and OATP1B1 substrate. In 12 RA patients, not treated with ACTEMRA, receiving 40 mg simvastatin, exposures of simvastatin and its metabolite, simvastatin acid, was 4- to 10-fold and 2-fold higher, respectively, than the exposures observed in healthy subjects. One week following administration of a single infusion of ACTEMRA (10 mg/kg), exposure of simvastatin and simvastatin acid decreased by 57% and 39%, respectively, to exposures that were similar or slightly higher than those observed in healthy subjects. Exposures of simvastatin and simvastatin acid increased upon withdrawal of ACTEMRA in RA patients. Selection of a particular dose of simvastatin in RA patients should take into account the potentially lower exposures that may result after initiation of ACTEMRA (due to normalization of CYP3A4) or higher exposures after discontinuation of ACTEMRA.
Omeprazole
Omeprazole is a CYP2C19 and CYP3A4 substrate. In RA patients receiving 10 mg omeprazole, exposure to omeprazole was approximately 2 fold higher than that observed in healthy subjects. In RA patients receiving 10 mg omeprazole, before and one week after ACTEMRA infusion (8 mg/kg), the omeprazole AUCinf decreased by 12% for poor (N=5) and intermediate metabolizers (N=5) and by 28% for extensive metabolizers (N=8) and were slightly higher than those observed in healthy subjects.
Dextromethorphan
Dextromethorphan is a CYP2D6 and CYP3A4 substrate. In 13 RA patients receiving 30 mg dextromethorphan, exposure to dextromethorphan was comparable to that in healthy subjects. However, exposure to its metabolite, dextrorphan (a CYP3A4 substrate), was a fraction of that observed in healthy subjects. One week following administration of a single infusion of ACTEMRA (8 mg/kg), dextromethorphan exposure was decreased by approximately 5%. However, a larger decrease (29%) in dextrorphan levels was noted after ACTEMRA infusion.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis. No long-term animal studies have been performed to establish the carcinogenicity potential of tocilizumab.
Mutagenesis. Tocilizumab was negative in the in vitro Ames bacterial reverse mutation assay and the in vitro chromosomal aberrations assay using human peripheral blood lymphocytes.
Impairment of Fertility. Fertility studies conducted in male and female mice using a murine analogue of tocilizumab showed no impairment of fertility.
14 CLINICAL STUDIES
The efficacy and safety of ACTEMRA was assessed in five randomized, double-blind, multicenter studies in patients > 18 years with active rheumatoid arthritis diagnosed according to American College of Rheumatology (ACR) criteria. Patients had at least 8 tender and 6 swollen joints at baseline. ACTEMRA was given intravenously every 4 weeks as monotherapy (Study I), in combination with methotrexate (MTX) (Studies II and III) or other disease-modifying anti-rheumatic drugs (DMARDs) (Study IV) in patients with an inadequate response to those drugs, or in combination with MTX in patients with an inadequate response to TNF antagonists (Study V).
Study I evaluated patients with moderate to severe active rheumatoid arthritis who had not been treated with MTX within 6 months prior to randomization, or who had not discontinued previous methotrexate treatment as a result of clinically important toxic effects or lack of response. In this study, 67% of patients were MTX-naïve, and over 40% of patients had rheumatoid arthritis < 2 years. Patients received ACTEMRA 8 mg/kg monotherapy or MTX alone (dose titrated over 8 weeks from 7.5 mg to a maximum of 20 mg weekly). The primary endpoint was the proportion of ACTEMRA patients who achieved an ACR20 response at Week 24.
Study II is an ongoing 2-year study with a planned interim analysis at week 24 that evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response to MTX. Patients received ACTEMRA 8 mg/kg, ACTEMRA 4 mg/kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). The primary endpoint at week 24 was the proportion of patients who achieved an ACR20 response.
Study III evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response to MTX. Patients received ACTEMRA 8 mg/kg, ACTEMRA 4 mg/kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). The primary endpoint was the proportion of patients who achieved an ACR20 response at week 24.
Study IV evaluated patients who had an inadequate response to their existing therapy, including one or more DMARDs. Patients received ACTEMRA 8 mg/kg or placebo every four weeks, in combination with the stable DMARDs. The primary endpoint was the proportion of patients who achieved an ACR20 response at week 24.
Study V evaluated patients with moderate to severe active rheumatoid arthritis who had an inadequate clinical response or were intolerant to one or more TNF antagonist therapies. The TNF antagonist therapy was discontinued prior to randomization. Patients received ACTEMRA 8 mg/kg, ACTEMRA 4 mg/kg, or placebo every four weeks, in combination with MTX (10 to 25 mg weekly). The primary endpoint was the proportion of patients who achieved an ACR20 response at week 24.
Clinical Response
The percentages of ACTEMRA-treated patients achieving ACR20, 50 and 70 responses are shown in Table 3. In all studies, patients treated with 8 mg/kg ACTEMRA had statistically significant ACR20, ACR50, and ACR70 response rates versus MTX- or placebo-treated patients at week 24.
Patients treated with ACTEMRA at a dose of 4 mg/kg in patients with inadequate response to DMARDs or TNF antagonist therapy had lower response rates compared to patients treated with ACTEMRA 8 mg/kg.
| Percent of Patients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Response Rate Week 24 | Study I | Study II | Study III | Study IV | Study V | ||||||||
| MTX | ACTEMRA 8 mg/kg | Placebo + MTX | ACTEMRA 4 mg/kg + MTX | ACTEMRA 8 mg/kg + MTX | Placebo + MTX | ACTEMRA 4 mg/kg + MTX | ACTEMRA 8 mg/kg + MTX | Placebo + DMARDs | ACTEMRA 8 mg/kg + DMARDs | Placebo + MTX | ACTEMRA 4 mg/kg + MTX | ACTEMRA 8 mg/kg + MTX | |
| N=284 | N=286 | N=393 | N=399 | N=398 | N=204 | N=213 | N=205 | N=413 | N=803 | N=158 | N=161 | N=170 | |
| ACR20 | |||||||||||||
| Responders | 53% | 70% | 27% | 51% | 56% | 27% | 48% | 59% | 25% | 61% | 10% | 30% | 50% |
| Weighted Difference %*
(95% CI)† | 19 (11, 27) | 23 (17, 29) | 29 (23, 35) | 23 (15, 32) | 32 (23, 41) | 35 (30, 40) | 25 (15, 36) | 46 (36, 56) |
|||||
| ACR50 | |||||||||||||
| Responders | 34% | 44% | 10% | 25% | 32% | 11% | 32% | 44% | 9% | 38% | 4% | 17% | 29% |
| Weighted Difference % *
(95% CI)† | 12 (4, 20) | 15 (9, 20) | 22 (16, 28) | 21 (13, 29) | 33 (25, 41) | 28 (23, 33) | 15 (5, 25) | 31 (21, 41) |
|||||
| ACR70 | |||||||||||||
| Responders | 15% | 28% | 2% | 11% | 13% | 2% | 12% | 22% | 3% | 21% | 1% | 5% | 12% |
| Weighted Difference % *
(95% CI)† | 14 (7, 22) | 8 (3, 13) | 10 (5, 15) | 11 (4, 18) | 20 (12, 27) | 17 (13, 21) | 4 (-6, 13) | 12 (3, 22) |
|||||
The results of the components of the ACR response criteria for Studies III and V are shown in Table 4. Similar results to Study III were observed in Studies I, II and IV.
| Study III | Study V | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTEMRA 4 mg/kg + MTX | ACTEMRA 8 mg/kg + MTX | Placebo + MTX | ACTEMRA 4 mg/kg + MTX | ACTEMRA 8 mg/kg + MTX | Placebo + MTX | |||||||
| N=213 | N=205 | N=204 | N=161 | N=170 | N=158 | |||||||
| Component (mean) | Baseline | Week 24* | Baseline | Week 24 * | Baseline | Week 24 | Baseline | Week 24 * | Baseline | Week 24 * | Baseline | Week 24 |
|
||||||||||||
| Number of tender joints (0-68) | 33 | 19 -7.0 (-10.0, -4.1) | 32 | 14.5 -9.6 (-12.6, -6.7) | 33 | 25 | 31 | 21 -10.8 (-14.6, -7.1) | 32 | 17 -15.1 (-18.8, -11.4) | 30 | 30 |
| Number of swollen joints (0-66) | 20 | 10 -4.2 (-6.1, -2.3) | 19.5 | 8 -6.2 (-8.1, -4.2) | 21 | 15 | 19.5 | 13 -6.2 (-9.0, -3.5) | 19 | 11 -7.2 (-9.9, -4.5) | 19 | 18 |
| Pain† | 61 | 33 -11.0 (-17.0, -5.0) | 60 | 30 -15.8 (-21.7, -9.9) | 57 | 43 | 63.5 | 43 -12.4 (-22.1, -2.1) | 65 | 33 -23.9 (-33.7, -14.1) | 64 | 48 |
| Patient global assessment† | 66 | 34 -10.9 (-17.1, -4.8) | 65 | 31 -14.9 (-20.9, -8.9) | 64 | 45 | 70 | 46 -10.0 (-20.3, 0.3) | 70 | 36 -17.4 (-27.8, -7.0) | 71 | 51 |
| Physician global assessment† | 64 | 26 -5.6 (-10.5, -0.8) | 64 | 23 -9.0 (-13.8, -4.2) | 64 | 32 | 66.5 | 39 -10.5 (-18.6, -2.5) | 66 | 28 -18.2 (-26.3, -10.0) | 67.5 | 43 |
| Disability index (HAQ)‡ | 1.64 | 1.01 -0.18 (-0.34, -0.02) | 1.55 | 0.96 -0.21 (-0.37, -0.05) | 1.55 | 1.21 | 1.67 | 1.39 -0.25 (-0.42, -0.09) | 1.75 | 1.34 -0.34 (-0.51, -0.17) | 1.70 | 1.58 |
| CRP (mg/dL) | 2.79 | 1.17 -1.30 (-2.0, -0.59) | 2.61 | 0.25 -2.156 (-2.86, -1.46) | 2.36 | 1.89 | 3.11 | 1.77 -1.34 (-2.5, -0.15) | 2.80 | 0.28 -2.52 (-3.72, -1.32) | 3.705 | 3.06 |
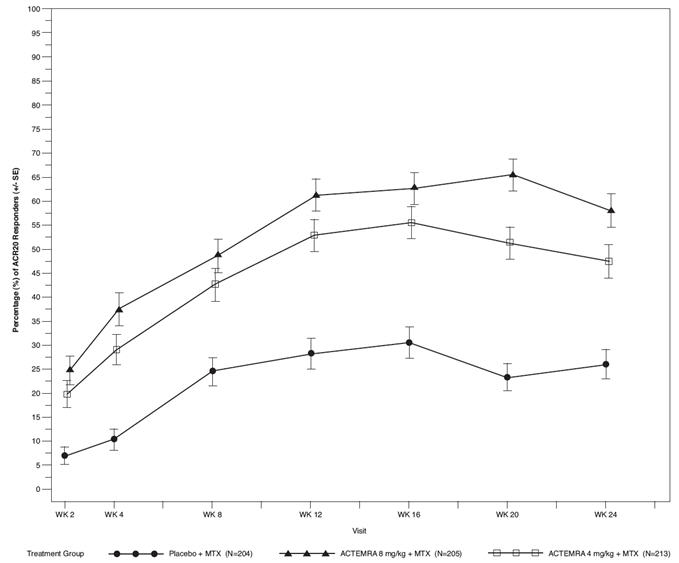
The percent of ACR20 responders by visit for Study III is shown in Figure 1. Similar responses were observed in studies I, II, IV, and V.
|
| Figure 1 Percent of ACR20 Responders by Visit for Study III (Inadequate Response to MTX)* |
 |
16 HOW SUPPLIED/STORAGE AND HANDLING
ACTEMRA (tocilizumab) is supplied in single-use vials as a preservative-free, sterile concentrate (20 mg/mL) solution for intravenous infusion. The following packaging configurations are available:
Individually packaged, single-use vials:
NDC 50242-135-01 providing 80 mg/4 mL
NDC 50242-136-01 providing 200 mg/10 mL
NDC 50242-137-01 providing 400 mg/20 mL
Box of 4 single-use vials:
NDC 50242-135-04 providing 80 mg/4 mL
NDC 50242-136-04 providing 200 mg/10 mL
NDC 50242-137-04 providing 400 mg/20 mL
Storage and Stability: Do not use beyond expiration date on the container. ACTEMRA must be refrigerated at 2ºC to 8ºC (36°F to 46°F). Do not freeze. Protect the vials from light by storage in the original package until time of use. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particles are observed, the solution should not be used.
17 PATIENT COUNSELING INFORMATION
17.1 Patient Counseling
Patients should be advised of the potential benefits and risks of ACTEMRA. Physicians should instruct their patients to read the Medication Guide before starting ACTEMRA therapy.
-
Infections:
Inform patients that ACTEMRA may lower their resistance to infections. Instruct the patient of the importance of contacting their doctor immediately when symptoms suggesting infection appear in order to assure rapid evaluation and appropriate treatment. -
Gastrointestinal Perforation:
Inform patients that some patients who have been treated with ACTEMRA have had serious side effects in the stomach and intestines. Instruct the patient of the importance of contacting their doctor immediately when symptoms of severe, persistent abdominal pain appear to assure rapid evaluation and appropriate treatment.
17.2 Medication Guide
MEDICATION GUIDE
ACTEMRA® (AC-TEM-RA)
(tocilizumab)
Read this Medication Guide before you start ACTEMRA and before each infusion. There may be new information. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about ACTEMRA?
ACTEMRA can cause serious side effects including:
1. Serious Infections
ACTEMRA is a medicine that affects your immune system. ACTEMRA can lower the ability of your immune system to fight infections. Some people have serious infections while taking ACTEMRA, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have died from these infections.
- Your doctor should test you for TB before starting ACTEMRA.
- Your doctor should monitor you closely for signs and symptoms of TB during treatment with ACTEMRA.
You should not start taking ACTEMRA if you have any kind of infection unless your healthcare provider says it is okay.
Before starting ACTEMRA, tell your healthcare provider if you:
- think you have an infection or have symptoms of an infection such as:
- fever, sweating, or chills
- muscle aches
- cough
- shortness of breath
- blood in phlegm
- weight loss
- warm, red, or painful skin or sores on your body
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
- feel very tired
- are being treated for an infection
- get a lot of infections or have infections that keep coming back
- have diabetes, HIV, or a weak immune system. People with these conditions have a higher chance for infections.
- have TB, or have been in close contact with someone with TB
- live or have lived, or have traveled to certain parts of the country (such as the Ohio and Mississippi River valleys and the Southwest) where there is an increased chance for getting certain kinds of fungal infections (histoplasmosis, coccidiomycosis, or blastomycosis). These infections may happen or become more severe if you use ACTEMRA. Ask your healthcare provider, if you do not know if you have lived in an area where these infections are common.
- have or have had hepatitis B.
After starting ACTEMRA, call your healthcare provider right away if you have any symptoms of an infection. ACTEMRA can make you more likely to get infections or make worse any infection that you have.
2. Tears (perforation) of the stomach or intestines.
- Before taking ACTEMRA, tell your healthcare provider if you have had diverticulitis (inflammation in parts of the large intestine) or ulcers in your stomach or intestines. Some people taking ACTEMRA get tears in their stomach or intestine. This happens most often in people who also take nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, or methotrexate.
- Tell your healthcare provider right away if you have fever and stomach-area pain that does not go away, and a change in your bowel habits.
3. Changes in certain laboratory test results. Your healthcare provider should do blood tests before you start receiving ACTEMRA and every 4 to 8 weeks during treatment to check for the following side effects of ACTEMRA:
- low neutrophil count. Neutrophils are white blood cells that help the body fight off bacterial infections.
- low platelet count. Platelets are blood cells that help with blood clotting and stop bleeding.
-
increase in certain liver function tests.
You should not receive ACTEMRA if your neutrophil or platelet counts are too low or your liver function tests are too high.
Your healthcare provider may stop your ACTEMRA treatment for a period of time or change your dose of medicine if needed because of changes in these blood test results.
You may also have changes in other laboratory tests, such as your blood cholesterol levels. Your healthcare provider should do blood tests to check your cholesterol levels 4 to 8 weeks after you start receiving ACTEMRA, and then every 6 months after that. Normal cholesterol levels are important to good heart health.
4. Cancer.
ACTEMRA may decrease the activity of your immune system. Medicines that affect the immune system may increase your risk of certain cancers. Tell your healthcare provider if you have ever had any type of cancer.
See "What are the possible side effects with ACTEMRA?" for more information about side effects.
What is ACTEMRA?
ACTEMRA is a prescription medicine called an Interleukin-6 (IL-6) receptor inhibitor. ACTEMRA is used to treat adults with moderately to severely active rheumatoid arthritis (RA) after at least one other medicine called a Tumor Necrosis Factor (TNF) antagonist has been used and did not work well.
It is not known if ACTEMRA is safe and effective in children.
What should I tell my healthcare provider before receiving ACTEMRA?
ACTEMRA may not be right for you. Before starting ACTEMRA, tell your healthcare provider if you:
- have an infection. See "What is the most important information I should know about ACTEMRA?"
- have liver problems
- have any stomach-area (abdominal) pain or been diagnosed with diverticulitis or ulcers in your stomach or intestines
- have or had a condition that affects your nervous system, such as multiple sclerosis
- have recently received or are scheduled to receive a vaccine. People who take ACTEMRA should not receive live vaccines. People taking ACTEMRA can receive non-live vaccines
- plan to have surgery or a medical procedure
- have any other medical conditions
- plan to become pregnant or are pregnant. It is not known if ACTEMRA will harm your unborn baby.
Pregnancy Registry: Genentech has a registry for pregnant women who take ACTEMRA. The purpose of this registry is to check the health of the pregnant mother and her baby. If you are pregnant or become pregnant while taking ACTEMRA, talk to your healthcare provider about how you can join this pregnancy registry or you may contact the registry at 1-877-311-8972 to enroll. - plan to breast-feed or are breast-feeding. You and your healthcare provider should decide if you will take ACTEMRA or breast-feed. You should not do both.
Tell your healthcare provider about all of the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. ACTEMRA and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- any other medicines to treat your RA. You should not take etanercept (Enbrel®), adalimumab (Humira®), infliximab (Remicade®), rituximab (Rituxan®), abatacept (Orencia®), anakinra (Kineret®), certolizumab (Cimzia®), or golimumab (Simponi®), while you are taking ACTEMRA. Taking ACTEMRA with these medicines may increase your risk of infection.
- medicines that affect the way certain liver enzymes work. Ask your healthcare provider if you are not sure if your medicine is one of these.
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How will I receive ACTEMRA?
- You will receive ACTEMRA from a healthcare provider through a needle placed in a vein in your arm (IV or intravenous infusion). The infusion will take about 1 hour to give you the full dose of medicine.
- You will receive a dose of ACTEMRA about every 4 weeks.
- If you miss a scheduled dose of ACTEMRA, ask your healthcare provider when to schedule your next infusion.
- While taking ACTEMRA, you may continue to use other medicines that help treat your rheumatoid arthritis such as methotrexate, non-steroidal anti-inflammatory drugs (NSAIDs) and prescription steroids, as instructed by your healthcare provider.
- Keep all of your follow-up appointments and get your blood tests as ordered by your healthcare provider.
What are the possible side effects with ACTEMRA?
ACTEMRA can cause serious side effects, including:
- See "What is the most important information I should know about ACTEMRA?"
-
Hepatitis B infection in people who carry the virus in their blood. If you are a carrier of the hepatitis B virus (a virus that affects the liver), the virus may become active while you use ACTEMRA. This happens with other biologic medicines used to treat RA. Your doctor may do blood tests before you start treatment with ACTEMRA and while you are using ACTEMRA. Tell your healthcare provider if you have any of the following symptoms of a possible hepatitis B infection:
- feel very tired
- skin or eyes look yellow
- little or no appetite
- vomiting
- clay-colored bowel movements
- fevers
- chills
- stomach discomfort
- muscle aches
- dark urine
- skin rash
- Nervous system problems. Multiple Sclerosis has been diagnosed rarely in people who take ACTEMRA. It is not known what effect ACTEMRA may have on some nervous system disorders.
-
Allergic Reactions. Serious allergic reactions can happen with ACTEMRA. These reactions may not happen with your first infusion, and may happen with future infusions of ACTEMRA. Tell your healthcare provider right away if you have any of the following signs of a serious allergic reaction:
- shortness of breath or trouble breathing
- skin rash
- swelling of the lips, tongue, or face
- chest pain
- feeling dizzy or faint
Common side effects of ACTEMRA include:
|
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of ACTEMRA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Genentech at 1-888-835-2555.
General information about ACTEMRA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. This Medication Guide summarizes the most important information about ACTEMRA.
If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about ACTEMRA that is written for health professionals.
For more information, go to www.ACTEMRA.com or call 1-800-ACTEMRA.
What are the ingredients in ACTEMRA?
Active ingredient: tocilizumab
Inactive ingredients: sucrose, polysorbate 80, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
ACTEMRA is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group.
Genentech, Inc.
A Member of the Roche Group
1 DNA Way
South San Francisco, CA 94080-4990
US License No. 1048
10094884
AAI_4877533_PI_062009_N_5
AAI_4877533_MG_062009_N_5
PI Issued: January 2010
© 2010 Genentech, Inc. All rights reserved.
Representative sample of labeling (see the HOW SUPPLIED section for complete listing):
PRINCIPAL DISPLAY PANEL - 10 mL Carton
NDC 50242-136-01
Actemra®
(tocilizumab)
Injection
200 mg/10 mL
(20 mg/ mL)
For Intravenous Infusion only after
dilution.
Single-Use Vial; Discard unused portion
ATTENTION PROVIDER: Each patient is
required to receive the enclosed
Medication Guide
No Preservative
Rx only
Genentech
| ACTEMRA
tocilizumab injection, solution, concentrate |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125276 | 01/08/2010 | |
| ACTEMRA
tocilizumab injection, solution, concentrate |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125276 | 01/08/2010 | |
| ACTEMRA
tocilizumab injection, solution, concentrate |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| BLA | BLA125276 | 01/08/2010 | |
| Labeler - Genentech, Inc. (080129000) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Chugai Pharma Manufacturing Co Ltd | 716464891 | ANALYSIS, API MANUFACTURE, MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| F. Hoffmann-La Roche Ltd | 482242971 | ANALYSIS, MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| F. Hoffmann-La Roche Ltd | 485244961 | MANUFACTURE | |