ACID REDUCER
-
ranitidine hydrochloride tablet
Safeway
----------
Safeway Inc. Acid Reducer 150 Drug FactsActive ingredient (in each tablet)
Ranitidine 150 mg (as ranitidine hydrochloride 168 mg)
Purpose
Acid reducer
Uses
- relieves heartburn associated with acid indigestion and sour stomach
- prevents heartburn associated with acid indigestion and sour stomach brought on by certain foods and beverages
Warnings
Allergy alert: Do not use if you are allergic to ranitidine or other acid reducers
Do not use
- if you have trouble or pain swallowing food, vomiting with blood, or bloody or black stools. These may be signs of a serious condition. See your doctor.
- with other acid reducers
- if you have kidney disease, except under the advice and supervision of a doctor
Ask a doctor before use if you have
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
Stop use and ask a doctor if
- your heartburn continues or worsens
- you need to take this product for more than 14 days
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over:
- to relieve symptoms, swallow 1 tablet with a glass of water
- to prevent symptoms, swallow 1 tablet with a glass of water 30 to 60 minutes before eating food or drinking beverages that cause heartburn
- can be used up to twice daily (do not take more than 2 tablets in 24 hours)
- children under 12 years: ask a doctor
Other information
- do not use if printed foil under cap is broken or missing
- avoid excessive heat or humidity
- store at 20° - 25°C (68° - 77°F)
- this product is sugar free
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, diethyl phthalate, FD&C yellow #6, hypromellose, iron oxide red, magnesium stearate, microcrystalline cellulose and titanium dioxide.
Questions or comments?
1-888-SAFEWAY
Principal Display Panel
Compare to Zantac 150® active ingredient
Maximum Strength
Acid Reducer 150
Ranitidine Tablets, 150 mg
Acid Reducer
Prevents and Relieves Heartburn Associated with Acid Indigestion and Sour Stomach
(# Doses) {replace "#" with the number of tablets in the package}
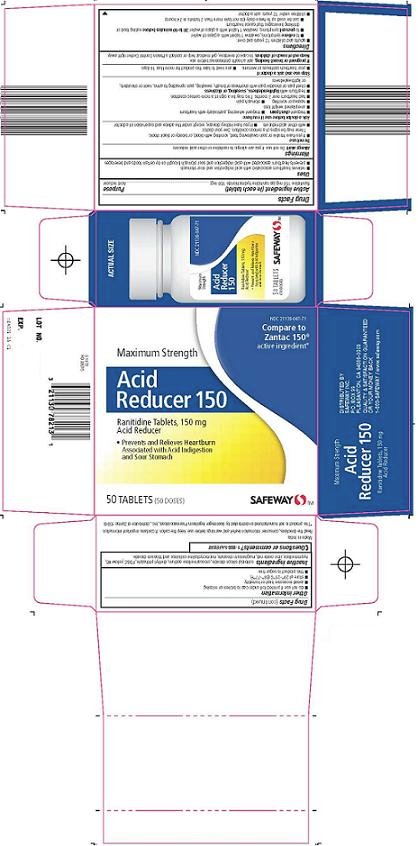
Acid Reducer 150 Carton
| ACID REDUCER
ranitidine tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA078653 | 02/20/2008 | |
| Labeler - Safeway (009137209) |