ACLARO PD HYDROQUINONE
-
hydroquinone emulsion
JSJ Pharmaceuticals
----------
Aclaro PDDescription
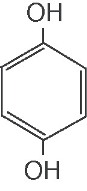
Hydroquinone is 1,4-benzenediol. Hydroquinone is structurallyrelated to monobenzone. Hydroquinone occurs as fine, white needles. The drug is
freely soluble in water and in alcohol with a pKa of 9.96. Chemically, hydroquinone
is designated as p–dihydroxybenzene; the empirical formula is C6H6O2; molecular
weight 110.1. The structural formula is:
ACTIVE INGREDIENT: hydroquinone USP 4%.
Other Ingredients: ascorbic acid, benzyl alcohol, butyl methoxydibensoyl
methane, C12-15 alkyl benzoate, cetearyl ethylhexanoate, cetyl alcohol, cetyl esters,
cetyl palmitate, DEA – cetyl phosphate, dimethicone, dimethylacrylamide/
acrylic acid/polystyrene ethyl methacrylate copolymer, disodium EDTA, ethylhexyl
methoxycinnamate, glycerine, glycolic acid, hydroxyl ethyl cellulose, phenoxyethanol,
propylene glycol (and) BHA (and) citric acid, purified water, sodium hydroxide solution,
stearic acid.
Clinical Pharmacology
Topical application of hydroquinone produces a reversibledepigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to
3-(3,4-dihydroxyphenyl) alanine (dopa)1 and suppression of other melanocyte metabolic
processes.2
Indications and Usage
Aclaro PD® is indicated for the gradual treatment of ultraviolet induced dyschromia anddiscoloration resulting from the use of oral contraceptives, pregnancy, hormone
replacement therapy, or skin trauma.
Contraindications
Aclaro PD® is contraindicated in any patient that has a
prior history of hypersensitivity or allergic reaction to hydroquinone or any of the
other ingredients. The safety of topical hydroquinone use during pregnancy or on
children (12 years and under) has not been established.
Warnings
A. Caution: Hydroquinone is a depigmenting agent which may produce unwantedcosmetic effects if not used as directed. The physician should be familiar with the
contents of this insert before prescribing or dispensing this medication.
B.Test for skin sensitivity before using Aclaro PD® (hydroquinone USP 4%)
bioadhesive emulsion by applying a small amount to an unbroken patch of skin and
check within 24 hours. Minor redness is not a contraindication, but where there
is itching, vesicle formation, or excessive inflammatory response, further treatment
is not advised. Close patient supervision is recommended. Contact with the eyes
should be avoided. If no lightening effect is noted after two months of treatment, use
of Aclaro PD® bioadhesive emulsion should be discontinued.
C. Sunscreen use is an essential aspect of hydroquinone therapy because even
minimal sunlight exposure sustains melanocyte activity. The sunscreens in
Aclaro PD® bioadhesive emulsion provide the necessary sun protection during
therapy. During and after the use of Aclaro PD® bioadhesive emulsion, sun exposure
should be limited or sun-protective clothing should be used to cover the treated areas
to prevent repigmentation.
D. Keep this and all medications out of the reach of children. In case of accidental
ingestion, contact a physician or poison control center immediately.
E. On rare occasions, a gradual blue-black darkening of the skin may occur. If this
occurs, the product should be discontinued and a physician contacted immediately.
Precautions
A. Pregnancy Category C: Animal reproduction studies have not been conducted with
topical hydroquinone. It is also not known whether hydroquinone can cause fetal harm
when used topically on a pregnant woman or affect reproductive capacity. It is not
known to what degree, if any, topical hydroquinone is absorbed systemically. Topical
hydroquinone should be used in pregnant women only when clearly indicated.
B. Nursing mothers: It is not known whether topical hydroquinone is absorbed or
excreted in human milk. Caution is advised when hydroquinone is used by a nursing
mother.
C. Pediatric usage: Safety and effectiveness
in pediatric patients below the age of 12
years have not been established.
Adverse Reactions
No systemic reactions have been reported. Occasionalcutaneous hypersensitivity (localized contact dermatitis) may occur, in which
case the medication should be discontinued and the physician notified immediately.
Overdosage
There have been no systemic reactions reported from the use of topical
hydroquinone. However, treatment should be limited to relatively small areas of the
body at one time, since some patients experience a transient skin reddening and
a mild burning sensation which does not preclude treatment.
Dosage and Administration
Aclaro PD®
bioadhesive emulsion should be applied to the affected areas twice daily, or as directed
by a physician. There is no recommended dosage for pediatric patients under 12
years of age except under the advice and supervision of a physician.
How Supplied
Aclaro PD® (hydroquinone USP 4%)bioadhesive emulsion is available in a:
1.5 ounce airless pump bottle NDC 68712-015-02
Store at controlled room temperature: 15˚-30˚ C (59˚–86˚ F)
Manufactured for: Innocutis
a JSJ Pharmaceuticals company
Charleston, SC 29401
Toll free: 1-800-499-4468
www.innocutis.com
www.Aclaro4.com
February 2011
U.S. Pat. No.: 5,942,243
| ACLARO PD
HYDROQUINONE
hydroquinone emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| unapproved drug other | 04/01/2011 | ||
| Labeler - JSJ Pharmaceuticals (615074866) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Sonar Products | 104283945 | manufacture | |