ACTAVIS INFANTS IBUPROFEN
-
ibuprofen suspension
Actavis Inc.
----------
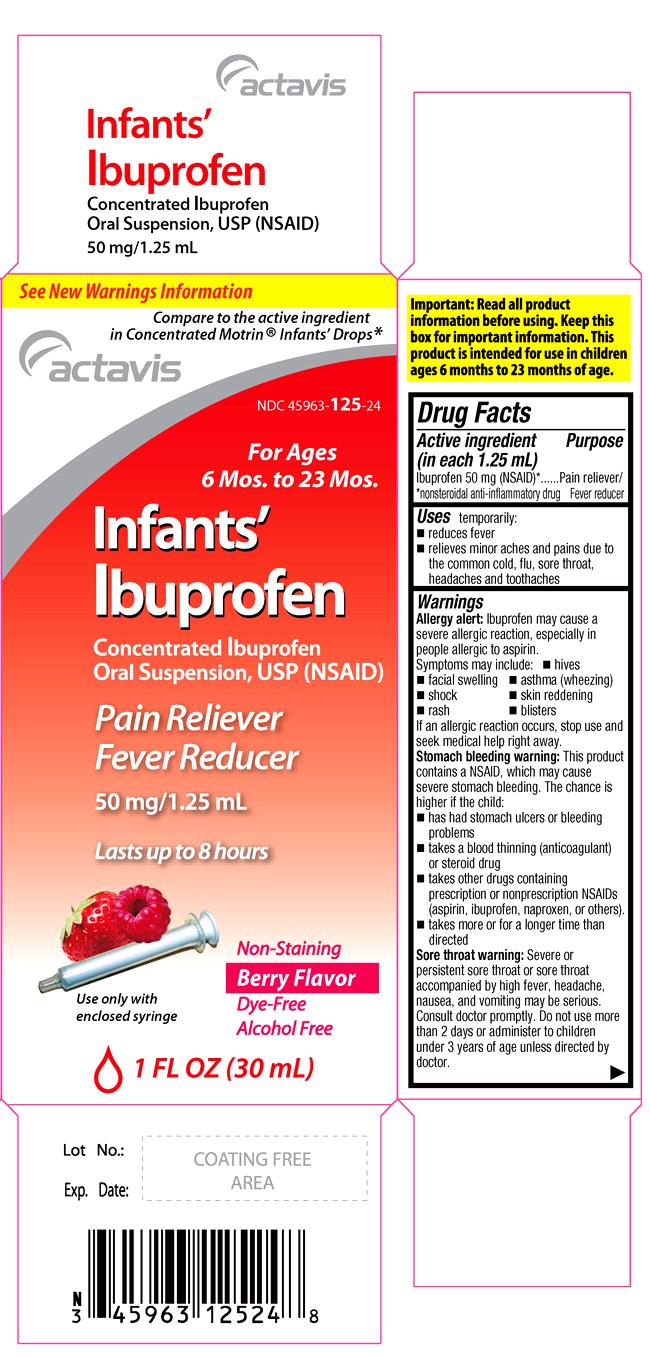
USES
temporarily:
- reduces fever
- relieves minor aches and pains due to the common cold, flu, sore throat, headaches and toothaches
WARNINGS
Allergy alert: Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning: This product contains a NSAID, which may cause severe stomach bleeding. The chance is higher if the child:
- has had stomach ulcers or bleeding problems
- takes a blood thinning (anticoagulant) or steroid drug
- takes other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- takes more or for a longer time than directed
Sore throat warning: Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
DO NOT USE
- if the child has ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
ASK A DOCTOR BEFORE USE IF
- stomach bleeding warning applies to your child
- child has a history of stomach problems, such as heartburn
- child has not been drinking fluids
- child has lost a lot of fluid due to vomiting or diarrhea
- child has high blood pressure, heart disease, liver cirrhosis, or kidney disease
- child is taking a diuretic
ASK A DOCTOR OR PHARMACIST BEFORE USE IF THE CHILD IS
- under a doctor’s care for any serious condition
- taking any other drug
WHEN USING THIS PRODUCT
- take with food or milk if stomach upset occurs
- the risk of heart attack or stroke may increase if you use more than directed or for longer than directed
STOP USE AND ASK A DOCTOR IF
- side effects occur. You may report side effects to FDA at 1-800-FDA-1088
- child experiences any of the following signs of stomach bleeding
- feels faint
- vomits blood
- has bloody or black stools
- has stomach pain that does not get better
- the child does not get any relief within the first day (24 hours) of treatment
- fever or pain gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
DIRECTIONS
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- shake well before using
- find right dose on chart below. If possible, use weight to dose; otherwise use age.
- measure with the dosing device provided. Do not use with any other device.
- dispense liquid slowly into the child’s mouth, toward the inner cheek
- if needed, repeat dose every 6 to 8 hours
- do not use more than 4 times a day
| Weight (lb) | Age (mos) | Dose (mL) |
| under 6 mos | ask a doctor | |
| 12 to 17 lbs | 6 to 11 mos | 1.25 mL |
| 18 to 23 lbs | 12 to 23 mos | 1.875 mL |
Other information
- store between 20 to 25°C (68 to 77°F)
- do not use if plastic bottle wrap imprinted “sealed for your protection” is broken or missing
- see bottom panel for lot number and expiration date
INACTIVE INGREDIENTS
carboxymethylcellulose sodium, citric acid anhydrous, glycerin, microcrystalline celullose, natural and artificial berry fruit punch type flavor, polysorbate-80, purified water, sodium benzoate, sorbitol solution, sucrose and xanthan gum.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
See New Warnings Information
Compare to the active ingredient in Concentrated Motrin® Infants’ Drops*
NDC 45963-125-24
For Ages 6 Mos. to 23 Mos.
Infants’ Ibuprofen
Concentrated Ibuprofen Oral Suspension, USP
(NSAID)
Pain Reliever
Fever Reducer
50 mg/1.25 mL
Lasts up to 8 hours
Use only with enclosed syringe
Non-Staining
Berry Flavor
Dye-Free
Alcohol Free
1 FL OZ (30 mL)

BERRY FLAVOR CARTON LABEL
| ACTAVIS INFANTS IBUPROFEN
concetrated ibuprofen suspension |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA079058 | 05/20/2010 | |
| Labeler - Actavis Inc. (099262404) |
Revised: 07/2011 Actavis Inc.