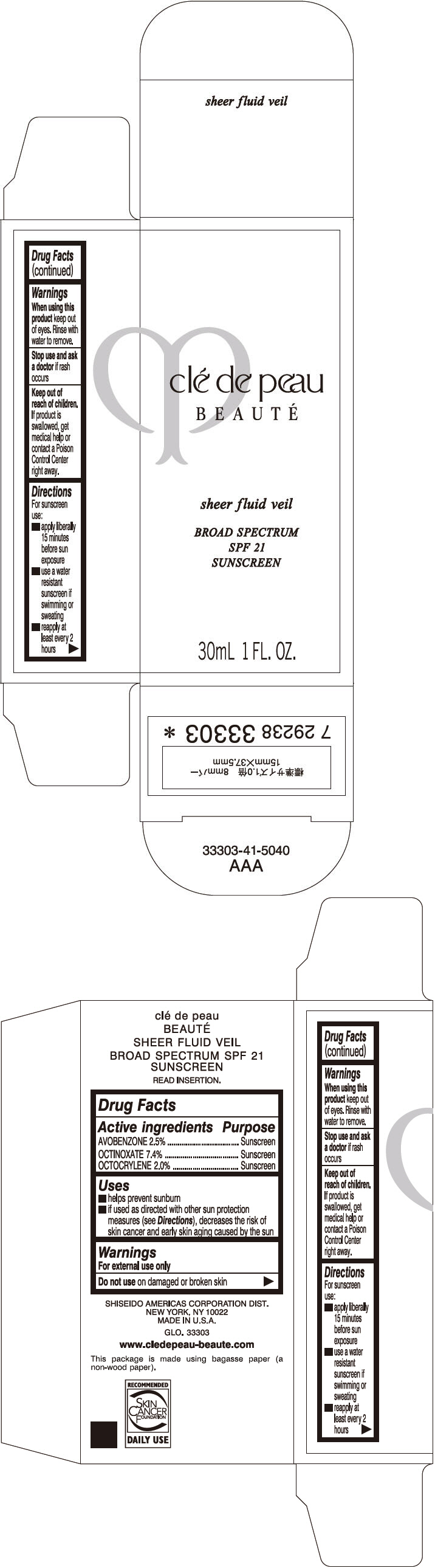
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
WATER∙SD ALCOHOL 40-B∙BUTYLENE GLYCOL∙XYLITOL∙DIMETHICONE∙TALC∙GLYCERIN∙POLYMETHYL METHACRYLATE∙PEG/PPG-19/19 DIMETHICONE∙HYDROGENATED POLYISOBUTENE∙XANTHAN GUM∙ERYTHRITOL∙SODIUM ACETYLATED HYALURONATE∙ROSA ROXBURGHII FRUIT EXTRACT∙DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER∙HYDROLYZED CONCHIOLIN PROTEIN∙HYDROLYZED SILK∙PPG-17∙DISODIUM EDTA∙PEG-60 GLYCERYL ISOSTEARATE∙TRIETHANOLAMINE∙CARBOMER∙ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER∙SODIUM METAPHOSPHATE∙BHT∙TOCOPHEROL∙SODIUM METABISULFITE∙POLYMETHYLSILSESQUIOXANE∙ALCOHOL∙FRAGRANCE∙IRON OXIDES∙MICA∙TITANIUM DIOXIDE∙