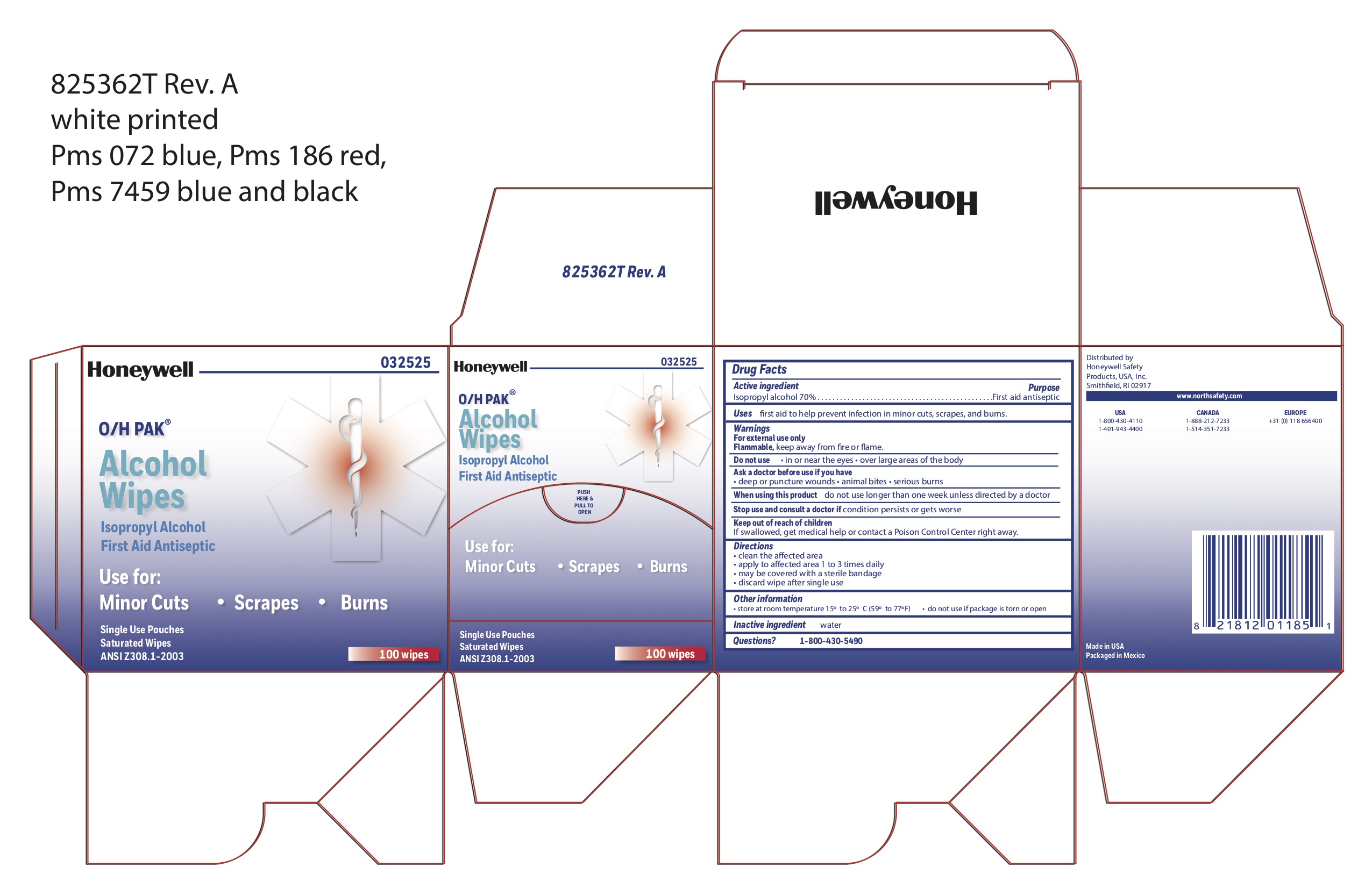
ALCOHOL WIPE- isopropyl alcohol swab
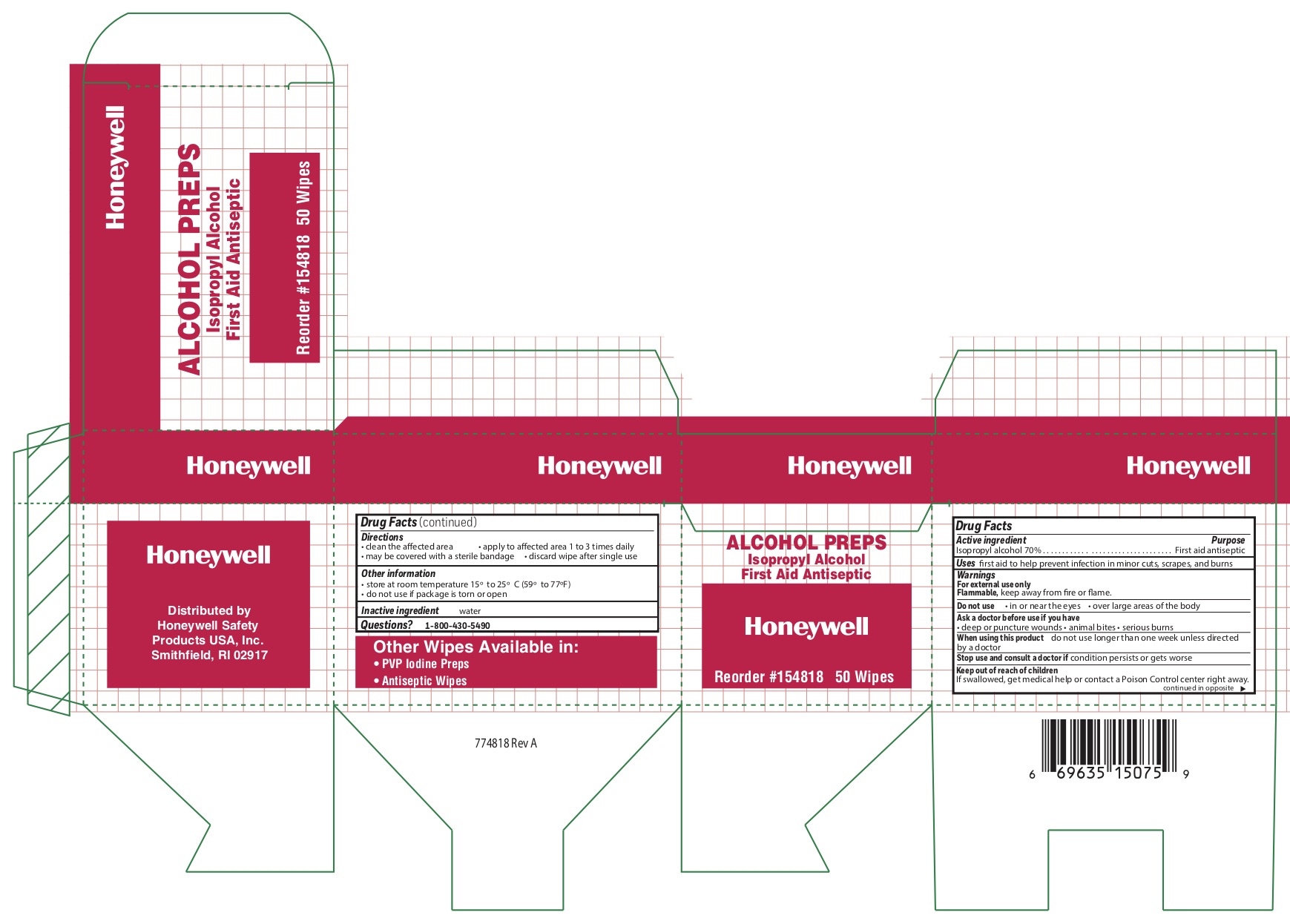
ALCOHOL PREP PAD- isopropyl alcohol swab
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Active ingredient
Isopropyl alcohol 70%
Purpose
First aid antiseptic
Uses
first aid to help prevent infection in minor cuts, scrapes, and burns
Warnings
For external use only
Flammable, keep away from fire and flame
Do not use
- in or near eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not use longer than 1 week unless directed by a doctor
Stop use and consult a doctor if
- condition persists or gets worse
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control center right away
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Other information
- store at room temperature 15
o to 25
o C (59
o to 77
oF)
- do not use if packet is torn or opened
Inactive ingredient
water
alcohol wipes
Alcohol wipes 10 ct

OH Pak