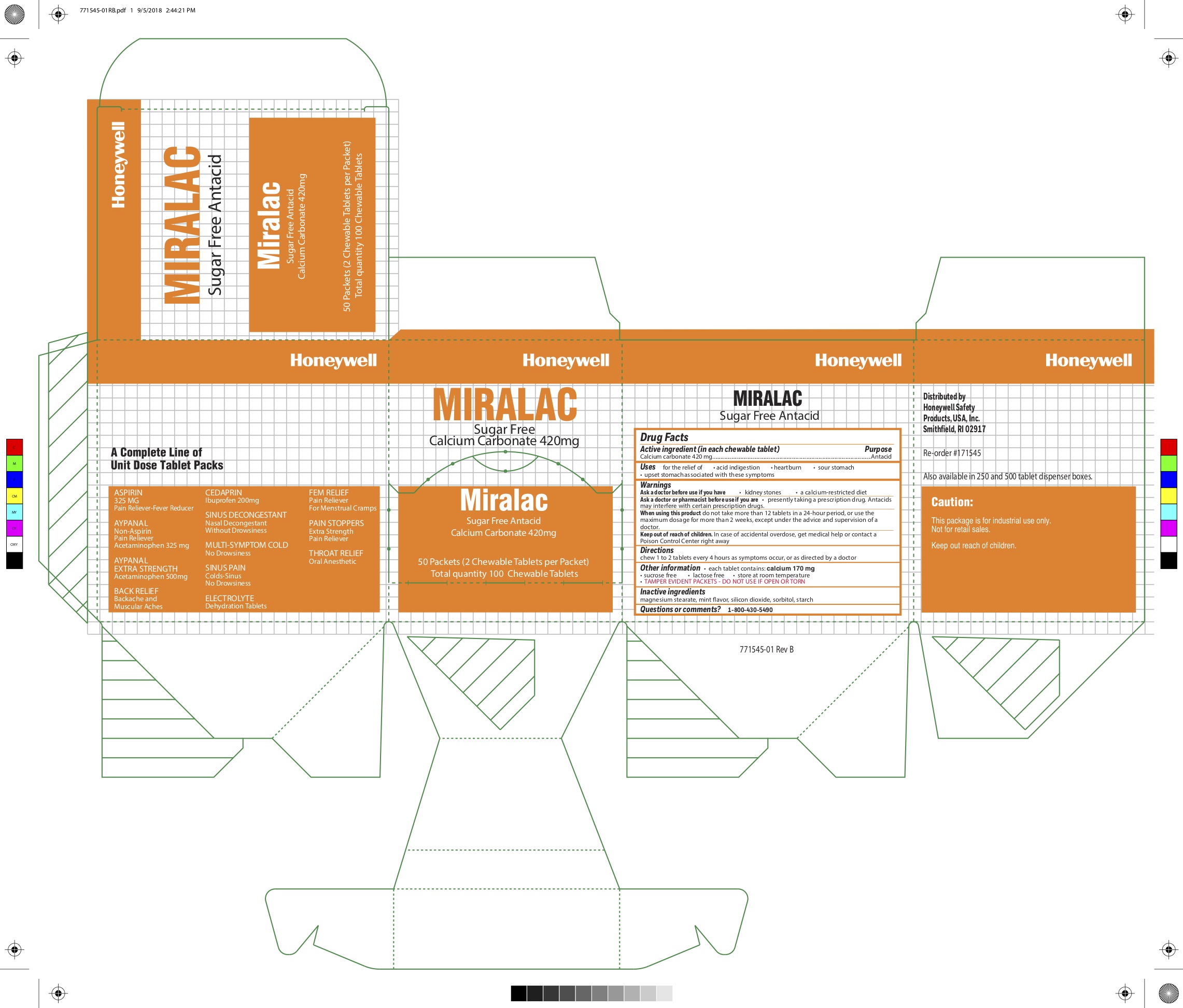
MIRALAC- calcium carbonate tablet
Honeywell Safety Products USA, Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-0303: Miralac
Uses
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Warnings
Ask a doctor before use if you are
presently taking a prescription drug. Antacids may interfere with certain prescription drugs
| MIRALAC
calcium carbonate tablet |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, Inc (118768815) |
Revised: 1/2024
Document Id: 0eca21e1-7b54-7974-e063-6294a90ac4b8
Set id: 7ccb324f-b12b-4867-87d5-b444e0c5b9a5
Version: 14
Effective Time: 20240112
Honeywell Safety Products USA, Inc