VISINE TEARS LONG LASTING DRY EYE RELIEF- glycerin, hypromelloses, and polyethylene glycol 400 solution/ drops
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
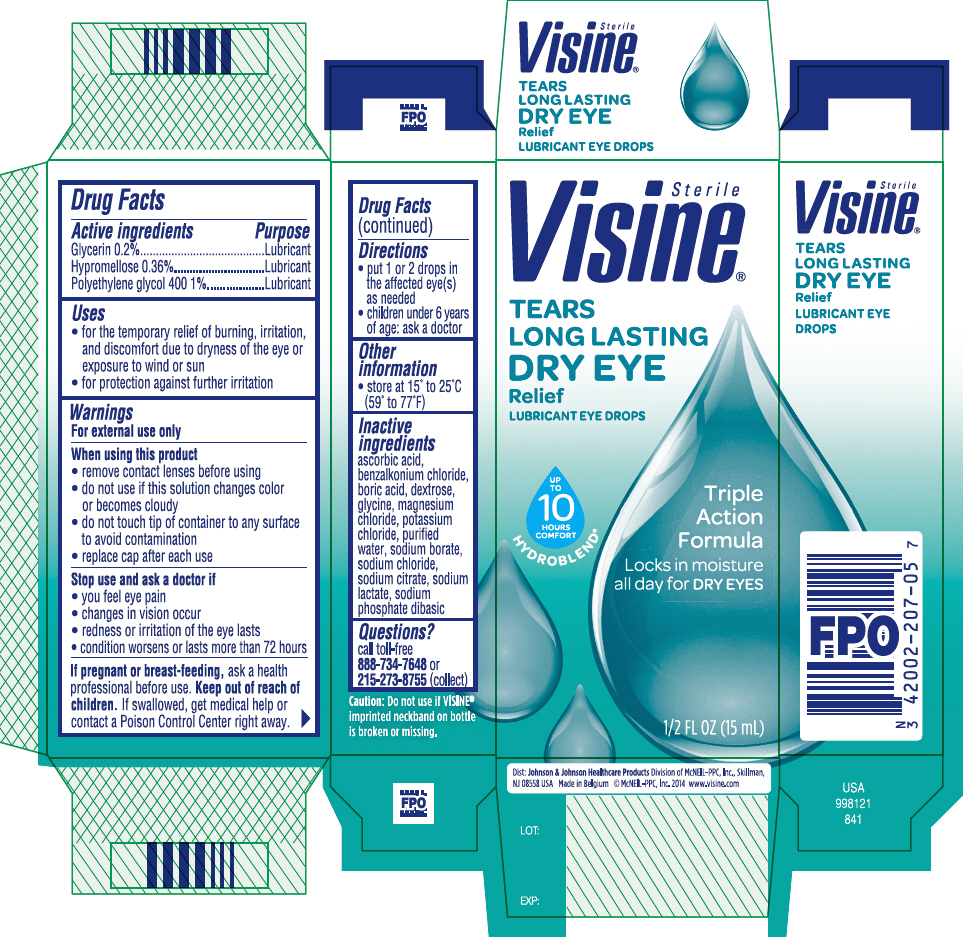
Visine® Tears® Long Lasting Dry Eye Relief Eye Drops
Uses
- for temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind or sun
- for protection against further irritation
Warnings
For external use only
When using this product
- remove contact lenses before using
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Directions
- put 1 or 2 drops in the affected eye(s) as needed
- children under 6 years of age: ask a doctor
| VISINE TEARS
LONG LASTING DRY EYE RELIEF
glycerin, hypromelloses, and polyethylene glycol 400 solution/ drops |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 3/2016
Document Id: 2ee86eda-a8ad-4683-b3fa-57017c332533
Set id: 92df7da6-366e-4de9-98ce-37f5d91f5997
Version: 4
Effective Time: 20160323
Johnson & Johnson Consumer Inc.