|
|
|||||||||||||||||||
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
AzaSite is indicated for the treatment of bacterial conjunctivitis caused by susceptible isolates of the following microorganisms:
- CDC coryneform group G*
- Haemophilus influenzae
- Staphylococcus aureus
- Streptococcus mitis group
- Streptococcus pneumoniae
*Efficacy for this organism was studied in fewer than 10 infections.
2 DOSAGE AND ADMINISTRATION
The recommended dosage regimen for the treatment of bacterial conjunctivitis is:
Instill 1 drop in the affected eye(s) twice daily, eight to twelve hours apart for the first two days and then instill 1 drop in the affected eye (s) once daily for the next five days.
3 DOSAGE FORMS AND STRENGTHS
5 mL bottle containing 2.5 mL of a 1% sterile topical ophthalmic solution.
4 CONTRAINDICATIONS
None
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
NOT FOR INJECTION. AzaSite is indicated for topical ophthalmic use only, and should not be administered systemically, injected subconjunctivally, or introduced directly into the anterior chamber of the eye.
5.2 Anaphylaxis and Hypersensitivity with Systemic Use of Azithromycin
In patients receiving systemically administered azithromycin, serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens Johnson Syndrome and toxic epidermal necrolysis have been reported rarely in patients on azithromycin therapy. Although rare, fatalities have been reported. While these reactions have not been observed with topical ophthalmic use of AzaSite, the potential for anaphylaxis or other hypersensitivity reactions should be considered since patients with a known hypersensitivity to azithromycin or erythromycin were excluded from study.
5.3 Growth of Resistant Organisms with Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If super-infection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and where appropriate, fluorescein staining.
5.4 Avoidance of Contact Lenses
Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in one clinical trial of a drug cannot be directly compared with the rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to AzaSite in 698 patients. The population was between 1 and 87 years old with clinical signs and symptoms of bacterial conjunctivitis. The most frequently reported ocular adverse reaction reported in patients receiving AzaSite was eye irritation. This reaction occurred in approximately 1-2% of patients. Other adverse reactions associated with the use of AzaSite were reported in less than 1% of patients and included: burning, stinging and irritation upon instillation, contact dermatitis, corneal erosion, dry eye, dysgeusia, nasal congestion, ocular discharge, punctate keratitis, and sinusitis.
7 DRUG INTERACTIONS
Drug interaction studies have not been conducted with AzaSite ophthalmic solution.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies have been performed in rats and mice at doses up to 200 mg/kg/d. The highest dose was associated with moderate maternal toxicity. These doses are estimated to be approximately 5000 times, the maximum human ocular daily dose of 2 mg. In the animal studies, no evidence of harm to the fetus due to azithromycin was found. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, azithromycin should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether azithromycin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when azithromycin is administered to a nursing woman.
8.4 Pediatric Use
The safety and effectiveness of AzaSite solution in pediatric patients below 1 year of age have not been established. The efficacy of AzaSite in treating bacterial conjunctivitis in pediatric patients one year or older has been demonstrated in controlled clinical trials. [see Clinical Studies (14)].
8.5 Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
11 DESCRIPTION
AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite® (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of approximately 290 mOsm/kg.
Preservative: 0.003% benzalkonium chloride. Inactives: mannitol, citric acid, sodium citrate, poloxamer 407, polycarbophil, edetate disodium (EDTA), sodium chloride, water for injection, and sodium hydroxide to adjust pH to 6.3.
Azithromycin is a macrolide antibiotic with a 15-membered ring. Its chemical name is (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6-aza-cyclopentadecan-15-one, and the structural formula is:
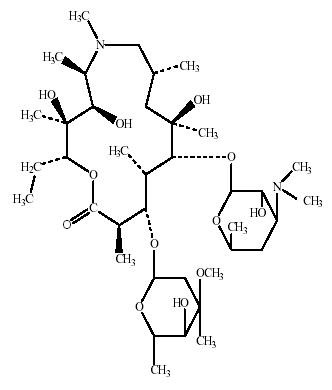
Azithromycin has a molecular weight of 749, and its empirical formula is C38H72N2O12.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Azithromycin is a macrolide antibiotic [see Clinical Pharmacology, Microbiology (12.4)].
12.3 Pharmacokinetics
The plasma concentration of azithromycin following ocular administration of AzaSite (azithromycin ophthalmic solution) in humans is unknown. Based on the proposed dose of one drop to each eye (total dose of 100 mcL or 1 mg) and exposure information from systemic administration, the systemic concentration of azithromycin following ocular administration is estimated to be below quantifiable limits (≤10 ng/mL) at steady-state in humans, assuming 100% systemic availability.
12.4 Microbiology
Azithromycin acts by binding to the 50S ribosomal subunit of susceptible microorganisms and interfering with microbial protein synthesis.
Azithromycin has been shown to be active against most isolates of the following microorganisms, both in vitro and clinically in conjunctival infections as described in the INDICATIONS AND USAGE section:
- CDC coryneform group G*
- Haemophilus influenzae
- Staphylococcus aureus
- Streptococcus mitis group
- Streptococcus pneumoniae
The following in vitro data are also available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of AzaSite in treating ophthalmological infections due to these microorganisms have not been established.
The following microorganisms are considered susceptible when evaluated using systemic breakpoints. However, a correlation between the in vitro systemic breakpoint and ophthalmological efficacy has not been established. This list of microorganisms is provided as an aid only in assessing the potential treatment of conjunctival infections. Azithromycin exhibits in vitro minimal inhibitory concentrations (MICs) of equal or less (systemic susceptible breakpoint) against most (≥90%) of isolates of the following ocular pathogens:
- Chlamydia pneumoniae
- Chlamydia trachomatis
- Legionella pneumophila
- Moraxella catarrhalis
- Mycoplasma hominis
- Mycoplasma pneumoniae
- Neisseria gonorrhoeae
- Peptostreptococcus species
- Streptococci (Groups C, F, G)
- Streptococcus pyogenes
- Streptococcus agalactiae
- Ureaplasma urealyticum
- Viridans group streptococci
*Efficacy for this organism was studied in fewer than 10 infections.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in standard laboratory tests: mouse lymphoma assay, human lymphocyte clastogenic assay, and mouse bone marrow clastogenic assay. No evidence of impaired fertility due to azithromycin was found in mice or rats that received oral doses of up to 200 mg/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) has been observed in some tissues of mice, rats, and dogs given multiple systemic doses of azithromycin. Cytoplasmic microvacuolation, which is likely a manifestation of phospholipidosis, has been observed in the corneas of rabbits given multiple ocular doses of AzaSite. This effect was reversible upon cessation of AzaSite treatment. The significance of this toxicological finding for animals and for humans is unknown.
14 CLINICAL STUDIES
In a randomized, vehicle-controlled, double-blind, multicenter clinical study in which patients were dosed twice daily for the first two days, then once daily on days 3, 4, and 5, AzaSite solution was superior to vehicle on days 6-7 in patients who had a confirmed clinical diagnosis of bacterial conjunctivitis. Clinical resolution was achieved in 63% (82/130) of patients treated with AzaSite versus 50% (74/149) of patients treated with vehicle. The p value for the comparison was 0.03 and the 95% confidence interval around the 13% (63%-50%) difference was 2% to 25%. The microbiological success rate for the eradication of the baseline pathogens was approximately 88% compared to 66% of patients treated with vehicle (p<.001, confidence interval around the 22% difference was 13% to 31%). Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
16 HOW SUPPLIED/STORAGE AND HANDLING
AzaSite is a sterile aqueous topical ophthalmic formulation of 1% azithromycin in a white, round, low-density polyethylene (LDPE) bottle, with a natural LDPE dropper tip, and a tan colored high density polyethylene (HDPE) eyedropper cap. A white tamper evident overcap is provided.
2.5 mL in 5 mL bottle containing a total of 25 mg of azithromycin
(NDC-31357-040-25)
Storage and Handling:
Store unopened bottle under refrigeration at 2°C to 8°C (36°F to 46°F). Once the bottle is opened, store at 2°C to 25°C (36°F to 77°F) for up to 14 days. Discard after the 14 days.
17 PATIENT COUNSELING INFORMATION
Patients should be advised to avoid contaminating the applicator tip by allowing it to touch the eye, fingers or other sources.
Patients should be directed to discontinue use and contact a physician if any signs of an allergic reaction occur.
Patients should be told that although it is common to feel better early in the course of the therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by AzaSite (azithromycin ophthalmic solution) or other antibacterial drugs in the future.
Patients should be advised not to wear contact lenses if they have signs or symptoms of bacterial conjunctivitis.
Patients are advised to thoroughly wash hands prior to using AzaSite.
Invert closed bottle (upside down) and shake once before each use. Remove cap with bottle still in the inverted position. Tilt head back, and with bottle inverted, gently squeeze bottle to instill one drop into the affected eye (s).
Inspire Pharmaceuticals Inc.
Licensee of InSite Vision Incorporated
Manufactured by Cardinal Health
U.S. PAT NO. 5,225,196; 5,192,535; 6,239,113; 6,569,443; 6,861,411; 7,056,893; and Patents Pending
AZA-0000
ISV06-42-0001
| AzaSite (Azithromycin) | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
Revised: 07/2007Inspire Pharmaceuticals, Inc.