AVEENO DIABETICS DRY SKIN RELIEF- dimethicone lotion
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
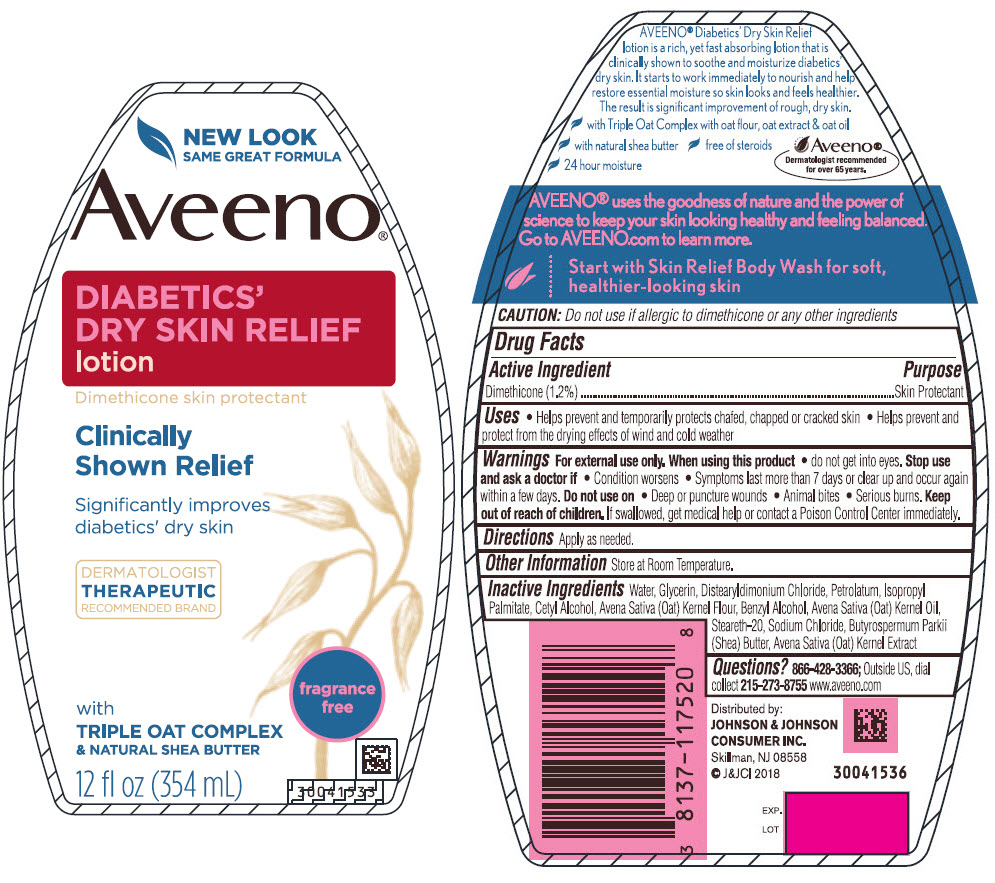
Aveeno ® DIABETICS' DRY SKIN RELIEF lotion
Uses
- Helps prevent and temporarily protects chafed, chapped or cracked skin
- Helps prevent and protect from the drying effects of wind and cold weather
Warnings
For external use only.
Inactive ingredients
Water, Glycerin, Distearyldimonium Chloride, Petrolatum, Isopropyl Palmitate, Cetyl Alcohol, Avena Sativa (Oat) Kernel Flour, Benzyl Alcohol, Avena Sativa (Oat) Kernel Oil, Steareth-20, Sodium Chloride, Butyrospermum Parkii (Shea) Butter, Avena Sativa (Oat) Kernel Extract
PRINCIPAL DISPLAY PANEL - 354 mL Bottle Label
NEW LOOK
SAME GREAT FORMULA
Aveeno ®
DIABETICS'
DRY SKIN RELIEF
lotion
Dimethicone skin protectant
Clinically
Shown Relief
Significantly improves
diabetics' dry skin
DERMATOLOGIST
THERAPEUTIC
RECOMMENDED BRAND
fragrance free
with
TRIPLE OAT COMPLEX
& NATURAL SHEA BUTTER
12 fl oz (354 mL)
| AVEENO DIABETICS DRY SKIN RELIEF
dimethicone lotion |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |