BANDA-SIL LIQUID GEL- argentum metallicum gel
AG Essence Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
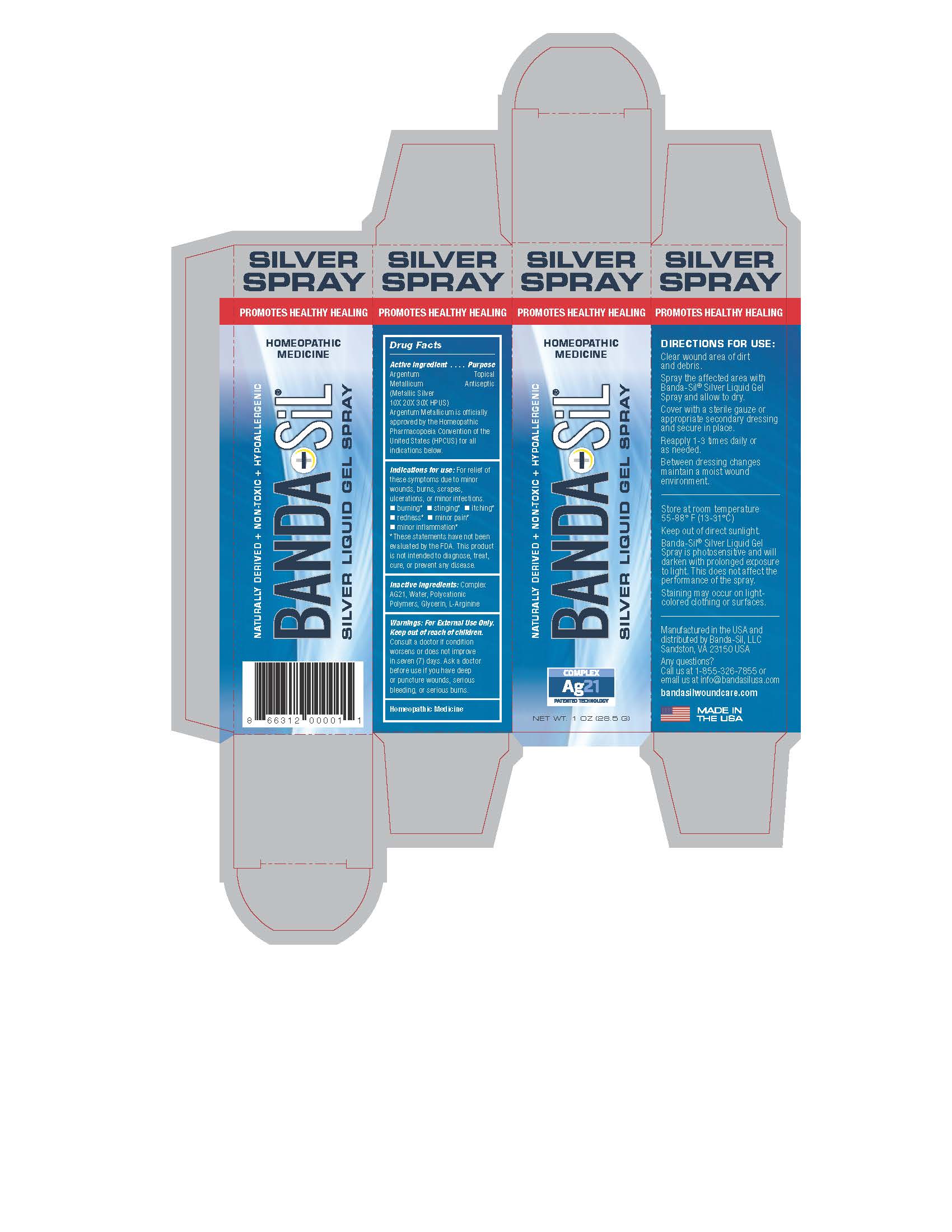
Directions For Use:
Clear wound area of dirt and debris.
Spray the affected area with Banda-Sil Silver Liquid Gel Spray and allow to dry.
Cover with a sterile gauze or appropriate secondary dressing and secure in place.
Reapply 1-3 times daily or as needed.
Between dressing changes maintain a moist wound environment.
Warnings:
For External Use Only. Keep out of reach of children.
Consult a doctor if condition worsens or does not improve in seven (7) days. Ask a doctor before use if you have deep or puncture wounds, serious bleeding, or servious burns.
| BANDA-SIL LIQUID GEL
argentum metallicum gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - AG Essence Inc (068562165) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AG Essence Inc | 068562165 | manufacture(72363-005) | |