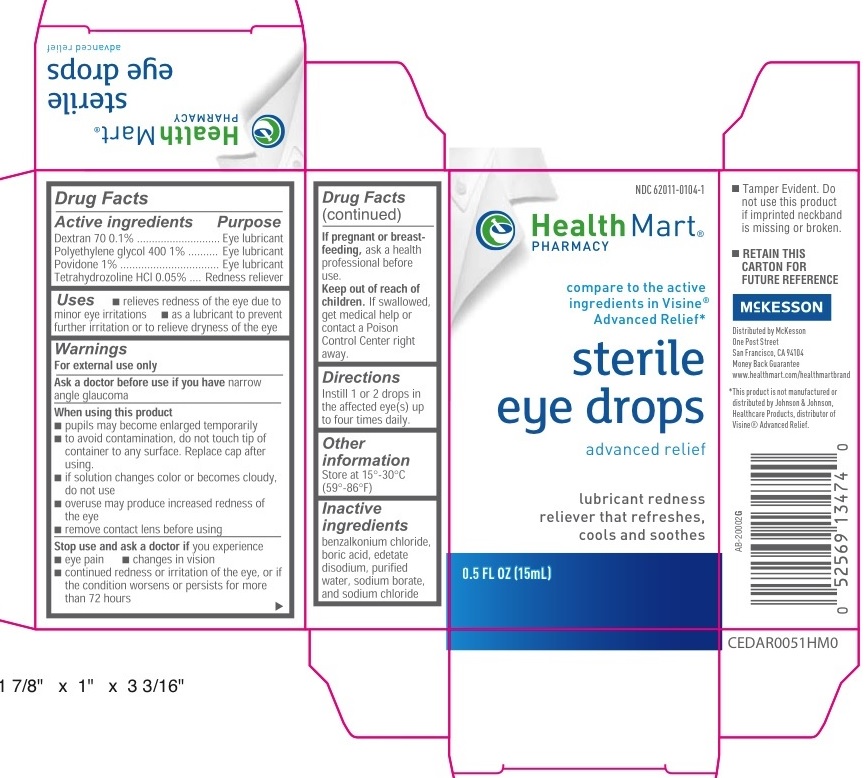
HEALTH MART EYE DROPS ADVANCED RELIEF- tetrahydrozoline hydrochloride, polyethylene glycol 400, dextran 70, povidone solution/ drops
Strategic Sourcing Services LLC
----------
Health Mart Eye Drops Advanced Relief (PLD)
Active ingredients
Dextran 70 0.1%
Polyethylene glycol 400 1%
Povidone 1%
Tetrahydrozoline HCl 0.05%
Purpose
Dextran 70..........Eye lubricant
Polyethylene glycol 400.....Eye lubricant
Povidone.............Eye lubricant
Tetrahydrozoline HCl.....Redness reliever
Uses
- relieves redness of the eye due to minor eye irritations
- as a lubricant to prevent further irritation or to relieve dryness of the eye
Warnings
For external use only
When using this product
- pupils may become enlarged temporarily
- to avoid contamination, do not touch tip of container to any surface. Replace cap after using
- if solution changes color or becomes cloudy, do not use
- overuse may product increased redness of the eye
- remove contact lens before using
Stop use and ask a doctor if you experience
- eye pain
- changes in vision
- continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours
| HEALTH MART EYE DROPS ADVANCED RELIEF
tetrahydrozoline hydrochloride, polyethylene glycol 400, dextran 70, povidone solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |
| Registrant - KC Pharmaceuticals, Inc. (174450460) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KC Pharmaceuticals, Inc. | 174450460 | manufacture(62011-0104) , pack(62011-0104) , label(62011-0104) | |
Revised: 1/2024
Document Id: 0e8a96d5-c3e1-c4aa-e063-6394a90a5a15
Set id: 5a6be263-ebda-45fb-aa39-138b27ef7f89
Version: 4
Effective Time: 20240109
Strategic Sourcing Services LLC