CALFARME FOAM CARE- benzalkonium chloride liquid
Calfarme (singapore) Pte.Ltd
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
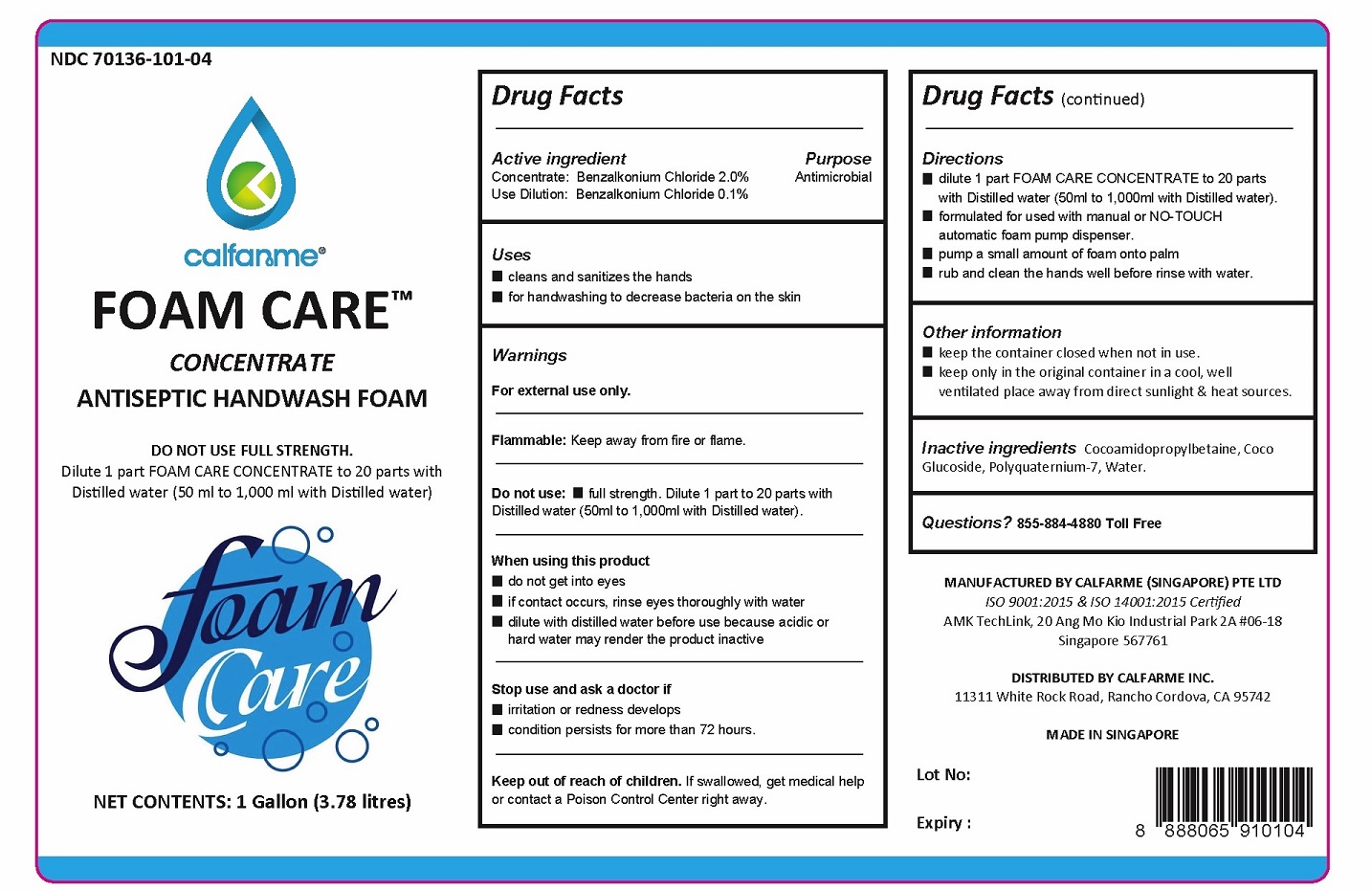
FOAM CARETMCONCENTRATE ANTISEPTIC HANDWASH FOAM
Warnings
For external use only.
Flammable: Keep away from fire or flame.
Do not use: ■ full strength. Dilute 1 part to 20 parts with Distilled water (50ml to 1,000ml with Distilled water).
When using this product
■ do not get into eyes
■ if contact occurs, rinse eyes thoroughly with water
■ dilute with distilled water before use because acidic or hard water may render the product inactive
Stop use and ask a doctor if
■ irritation or redness develops
■ condition persists for more than 72 hours.
Directions
■ dilute 1 part FOAM CARE CONCENTRATE to 20 parts with Distilled water (50ml to 1,000ml with Distilled water).
■ formulated for used with manual or NO-TOUCH automatic foam pump dispenser.
■ pump a small amount of foam onto palm
■ rub and clean the hands well before rinse with water.
Other Information
■ keep the container closed when not in use.
■ keep only in the original container in a cool, well ventilated place away from direct sunlight & heat sources.
| CALFARME
FOAM CARE
benzalkonium chloride liquid |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Calfarme (singapore) Pte.Ltd (595222746) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Calfarme (singapore) Pte.Ltd | 595222746 | manufacture(70136-101) | |