FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Insulin Aspart is indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
2 DOSAGE AND ADMINISTRATION
2.1 Important Preparation and Administration Instructions
- Always check insulin labels before administration. This product is NovoLog (insulin aspart) [seeWarnings and Precautions ( 5.4)] .
- Inspect Insulin Aspart visually before use. It should appear clear and colorless. Do not use Insulin Aspart if particulate matter or coloration is seen.
- In patients with visual impairment, use:
- Insulin Aspart FlexPen with caution in those who may rely on audible clicks to dial
- their dose.
- PenFill cartridges with caution.
- Do notmix Insulin Aspart with other insulins when administering using a continuous subcutaneous infusion pump.
2.2 Preparation and Administration Instructions for the Approved Routes of Administration
Subcutaneous Injection
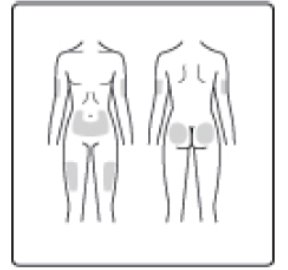
- Inject Insulin Aspart subcutaneously within 5-10 minutes before a meal into the abdominal area, thigh, buttocks or upper arm.
- Rotate injection sites within the same region from one injection to the next to reduce the risk of lipodystrophy and localized cutaneous amyloidosis. Do not inject into areas of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions ( 5.2) and Adverse Reactions ( 6.1, 6.3)].
- Dial the Insulin Aspart FlexPen dials in 1-unit increments.
- Generally use Insulin Aspart (administered by subcutaneous injection) in regimens with an intermediate- or long-acting insulin.
- May dilute this Insulin Aspart product with Insulin Diluting Medium for NovoLog for subcutaneous injection. Diluting one part Insulin Aspart to:
- Nine parts diluent will yield a concentration one-tenth that of Insulin Aspart (equivalent to U-10).
- One part diluent will yield a concentration one-half that of Insulin Aspart (equivalent to U-50).
Continuous Subcutaneous Infusion (Insulin Pump)
- Can use this Insulin Aspart product with the continuous subcutaneous insulin infusion pumps labeled for use with NovoLog (insulin aspart). Refer to the insulin pump user manual to see if NovoLog can be used. Use Insulin Aspart in accordance with the insulin pump system’s instructions for use.
- Train patients using continuous subcutaneous insulin infusion pump therapy to administer insulin by injection and have alternate insulin therapy available in case of pump failure.
- Administer Insulin Aspart by continuous subcutaneous infusion in a region recommended in the instructions from the pump manufacturer. Rotate infusion sites within the same region to reduce the risk of lipodystrophy or localized cutaneous amyloidosis [see Warnings and Precautions (5.2) and Adverse Reactions (6.1, 6.3)].
- Instruct patients to follow healthcare provider recommendations when setting basal and meal time infusion rate.
- Change the Insulin Aspart in the reservoir at least every 7 days or according to the pump user manual, whichever is shorter. Follow the NovoLog-specific information for in-use time because NovoLog-specific information may differ from general pump manual instructions.
- Change the infusion set and the infusion set insertion site according to the manufacturer’s user manual.
- Do notdilute or mix Insulin Aspart when administering by continuous subcutaneous infusion.
- Do notexpose Insulin Aspart in the pump reservoir to temperatures greater than 98.6°F (37°C).
Intravenous Administration
- Administer Insulin Aspart intravenously only under medical supervision with close monitoring of blood glucose and potassium levels to avoid hypoglycemia and hypokalemia [see Warnings and Precautions (5.3, 5.6) and How Supplied/Storage and Handling (16.2)].
- Dilute Insulin Aspart to concentrations from 0.05 unit/mL to 1 unit/mL insulin aspart in infusion systems using polypropylene infusion bags. Insulin Aspart is stable in infusion fluids such as 0.9% Sodium Chloride Injection, USP.
2.3 Dosage Recommendations
- Individualize the dosage of Insulin Aspart based on the route of administration, the patient’s metabolic needs, blood glucose monitoring results and glycemic control goal.
- Dosage adjustments may be needed with changes in physical activity, changes in meal patterns (i.e., macronutrient content or timing of food intake), changes in renal or hepatic function or during acute illness [see Warnings and Precautions ( 5.2, 5.3) and Use in Specific Populations ( 8.6, 8.7)] .
- When switching from another insulin to Insulin Aspart, a different dosage of Insulin Aspart may be needed [see Warnings and Precautions ( 5.2)] .
- During changes to a patient’s insulin regimen, increase the frequency of blood glucose monitoring [see Warnings and Precautions (5.2)].
2.4 Dosage Modifications for Drug Interactions
Dosage modification may be needed when Insulin Aspart is used concomitantly with certain drugs [see Drug Interactions (7)].
2.5 Instructions for Mixing Insulin Aspart with Other Insulins
The table below includes instructions regarding mixing Insulin Aspart with other insulins.
|
Subcutaneous injection route |
•If Insulin Aspart is mixed with NPH insulin, withdraw Insulin Aspart into the syringe first and inject immediately after mixing. |
|
Continuous subcutaneous infusion route (Insulin Pump) |
Do notmix Insulin Aspart with any other insulin. |
3 DOSAGE FORMS AND STRENGTHS
Injection: 100 units/mL (U-100) is a clear and colorless solution available as:
- 10 mL multiple-dose vial
- 3 mL single-patient-use PenFill prefilled cartridges for the 3 mL PenFill cartridge delivery device with NovoFine ®disposable needles
- 3 mL single-patient-use FlexPen prefilled pen
5 WARNINGS AND PRECAUTIONS
5.1 Never Share a Insulin Aspart FlexPen, PenFill Cartridge, or PenFill Cartridge Device between Patients
Insulin Aspart FlexPen, PenFill cartridge, and PenFill cartridge devices should never be shared between patients, even if the needle is changed. Patients using Insulin Aspart vials must never share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens.
5.2 Hyperglycemia or Hypoglycemia with Changes in Insulin Regimen
Changes in an insulin regimen (e.g., insulin strength, manufacturer, type, injection site or method of administration) may affect glycemic control and predispose to hypoglycemia [see Warnings and Precautions ( 5.3)] or hyperglycemia. Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis have been reported to result in hyperglycemia; and a sudden change in the injection site (to an unaffected area) has been reported to result in hypoglycemia [see Adverse Reactions ( 6.1, 6.3)]. Make any changes to a patient’s insulin regimen under close medical supervision with increased frequency of blood glucose monitoring. Advise patients who have repeatedly injected into areas of lipodystrophy or localized cutaneous amyloidosis to change the injection site to unaffected areas and closely monitor for hypoglycemia. For patients with type 2 diabetes, dosage adjustments of concomitant anti-diabetic products may be needed.
5.3 Hypoglycemia
Hypoglycemia is the most common adverse reaction of all insulins, including Insulin Aspart. Severe hypoglycemia can cause seizures, may lead to unconsciousness, may be life threatening or cause death. Hypoglycemia can impair concentration ability and reaction time; this may place an individual and others at risk in situations where these abilities are important (e.g., driving or operating other machinery).
Hypoglycemia can happen suddenly and symptoms may differ in each individual and change over time in the same individual. Symptomatic awareness of hypoglycemia may be less pronounced in patients with longstanding diabetes in patients with diabetic nerve disease, in patients using medications that block the sympathetic nervous system (e.g., beta-blockers) [see Drug Interactions ( 7)] , or in patients who experience recurrent hypoglycemia.
Risk Factors for Hypoglycemia
The risk of hypoglycemia after an injection is related to the duration of action of the insulin and, in general, is highest when the glucose lowering effect of the insulin is maximal. As with all insulins, the glucose lowering effect time course of Insulin Aspart may vary in different individuals or at different times in the same individual and depends on many conditions, including the area of injection as well as the injection site blood supply and temperature [see Clinical Pharmacology ( 12.2)] . Other factors which may increase the risk of hypoglycemia include changes in meal pattern (e.g., macronutrient content or timing of meals), changes in level of physical activity, or changes to concomitantly administered medication [see Drug Interactions ( 7)] . Patients with renal or hepatic impairment may be at higher risk of hypoglycemia [see Use in Specific Populations ( 8.6, 8.7)].
Risk Mitigation Strategies for Hypoglycemia
Patients and caregivers must be educated to recognize and manage hypoglycemia. Self-monitoring of blood glucose plays an essential role in the prevention and management of hypoglycemia; increased frequency of blood glucose monitoring is recommended. In patients at higher risk for hypoglycemia and patients who have reduced symptomatic awareness of hypoglycemia; increased frequency of blood glucose monitoring is recommended.
5.4 Hypoglycemia Due to Medication Errors
Accidental mix-ups between insulin products have been reported. To avoid medication errors between this Insulin Aspart product and other insulins, instruct patients to always check the insulin label before each injection.
5.5 Hypersensitivity Reactions
Severe, life-threatening, generalized allergy, including anaphylaxis, can occur with insulins, including Insulin Aspart. If hypersensitivity reactions occur, discontinue Insulin Aspart; treat per standard of care and monitor until symptoms and signs resolve [see Adverse Reactions ( 6)]. Insulin Aspart is contraindicated in patients who have had hypersensitivity reactions to insulin aspart or one of the excipients [see Contraindications ( 4)].
5.6 Hypokalemia
All insulins, including Insulin Aspart, can cause a shift in potassium from the extracellular to intracellular space, possibly leading to hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Monitor potassium levels in patients at risk for hypokalemia if indicated (e.g., patients using potassium-lowering medications, patients taking medications sensitive to serum potassium concentration).
5.7 Fluid Retention and Heart Failure with Concomitant Use of PPAR-gamma Agonists
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor (PPAR)-gamma agonists, can cause dose-related fluid retention, particularly when used in combination with insulin. Fluid retention may lead to or exacerbate heart failure. Patients treated with insulin, including Insulin Aspart, and a PPAR-gamma agonist should be observed for signs and symptoms of heart failure. If heart failure develops, it should be managed according to current standards of care, and discontinuation or dose reduction of the PPAR-gamma agonist must be considered.
5.8 Hyperglycemia and Ketoacidosis Due to Insulin Pump Device Malfunction
Malfunction of the insulin pump or insulin infusion set or insulin degradation can rapidly lead to hyperglycemia and ketoacidosis. Prompt identification and correction of the cause of hyperglycemia or ketosis is necessary. Interim subcutaneous injections with Insulin Aspart may be required. Patients using continuous subcutaneous insulin infusion pump therapy must be trained to administer insulin by injection and have alternate insulin therapy available in case of pump failure [see How Supplied/Storage and Handling ( 16.2) and Patient Counseling Information ( 17)] .
6 ADVERSE REACTIONS
- The following adverse reactions are also discussed elsewhere:
- Hypoglycemia [seeWarnings and Precautions ( 5.3)]
- Hypoglycemia Due to Medication Errors [see Warnings and Precautions (5.4)]
- Hypersensitivity reactions [see Warnings and Precautions (5.5)]
- Hypokalemia [seeWarnings and Precautions ( 5.6)]
6.1 Clinical Trial Experience
- Because clinical trials are conducted under widely varying designs, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice. The safety of Insulin Aspart was evaluated in two treat-to-target trials of 6 months duration, conducted in patients with type 1 diabetes or type 2 diabetes [seeClinical Studies ( 14)].
- The data in Table 1 reflect the exposure of 596 patients with type 1 diabetes to Insulin Aspart in one clinical trial with a mean exposure duration to Insulin Aspart of 24 weeks. The mean age was 39 years. Fifty-one percent were male, 94% were Caucasian, 2% were Black and 4% were other races. The mean body mass index (BMI) was 25.6 kg/m 2. The mean duration of diabetes was 15.7 years and the mean HbA 1cat baseline was 7.9%.
- The data in Table 2 reflect the exposure of 91 patients with type 2 diabetes to Insulin Aspart in one clinical trial with a mean exposure duration to Insulin Aspart of 24 weeks. The mean age was 57 years. Sixty-three percent were male, 76% were Caucasian, 9% were Black and 15% were other races. The mean BMI was 29.7 kg/m 2. The mean duration of diabetes was 12.7 years and the mean HbA 1cat baseline was 8.1%.
Common adverse reactions were defined as events that occurred in ≥5%, excluding hypoglycemia, of the population studied. Common adverse events that occurred at the same rate or greater for Insulin Aspart-treated patients than in comparator-treated patients during clinical trials in patients with type 1 diabetes mellitus and type 2 diabetes mellitus (other than hypoglycemia) are listed in Table 1 and Table 2, respectively.
Table 1: Adverse reactions that occurred in≥ 5% of Type 1 Diabetes Mellitus Adult Patients treated with Insulin Aspart and at the same rate or greater on Insulin Aspart than on comparator
| Insulin Aspart + NPH (%)
(n= 596) | Regular Human Insulin + NPH (%)
(n= 286) |
|
|---|---|---|
|
Headache |
12 |
10 |
|
Injury accidental |
11 |
10 |
|
Nausea |
7 |
5 |
|
Diarrhea |
5 |
3 |
Table 2: Adverse reactions that occurred in≥ 5% of Type 2 Diabetes Mellitus Adult Patients treated with Insulin Aspart and at the same rate or greater on Insulin Aspart than on comparator
| Insulin Aspart + NPH (%)
(n= 91) | Human Regular Insulin + NPH (%)
(n= 91) |
|
|---|---|---|
|
Hyporeflexia |
11 |
7 |
|
Onychomycosis |
10 |
5 |
|
Sensory disturbance |
9 |
7 |
|
Urinary tract infection |
8 |
7 |
|
Chest pain |
5 |
3 |
|
Headache |
5 |
3 |
|
Skin disorder |
5 |
2 |
|
Abdominal pain |
5 |
1 |
|
Sinusitis |
5 |
1 |
Severe Hypoglycemia
Hypoglycemia is the most commonly observed adverse reaction in patients using insulin, including Insulin Aspart .The rates of reported hypoglycemia depend on the definition of hypoglycemia used, diabetes type, insulin dose, intensity of glucose control, background therapies, and other intrinsic and extrinsic patient factors. For these reasons, comparing rates of hypoglycemia in clinical trials for Insulin Aspart with the incidence of hypoglycemia for other products may be misleading and also, may not be representative of hypoglycemia rates that will occur in clinical practice.
Severe hypoglycemia was defined as hypoglycemia associated with central nervous system symptoms and requiring the intervention of another person or hospitalization. The incidence of severe hypoglycemia in:
- Adult and pediatric patients with type 1 diabetes mellitus who received subcutaneous Insulin Aspart was 17% at 24 weeks and 6% at 24 weeks, respectively [see Clinical Studies (14)] .
- Adult patients with type 2 diabetes mellitus who received subcutaneous Insulin Aspart was 10% at 24 weeks.
- Adult and pediatric patients with type 1 diabetes mellitus, who received Insulin Aspart via continuous subcutaneous insulin infusion by external pump was 2% at 16 weeks and 10% at 16 weeks respectively.
No severe hypoglycemic episodes were reported in adult patients with type 2 diabetes mellitus receiving Insulin Aspart via continuous subcutaneous insulin infusion by external pump at 16 weeks.
Allergic Reactions
Some patients taking insulin, including Insulin Aspart have experienced erythema, local edema, and pruritus at the site of injection. These conditions were usually self-limiting. Severe cases of generalized allergy (anaphylaxis) have been reported.
Adverse Reactions Associated with Insulin Initiation and Glucose Control Intensification
Intensification or rapid improvement in glucose control has been associated with a transitory, reversible ophthalmologic refraction disorder, worsening of diabetic retinopathy, and acute painful peripheral neuropathy. However, long-term glycemic control decreases the risk of diabetic retinopathy and neuropathy.
Lipodystrophy
Administration of insulin, including Insulin Aspart, subcutaneously and via subcutaneous insulin infusion by external pump, has resulted in lipoatrophy (depression in the skin) or lipohypertrophy (enlargement or thickening of tissue) in some patients [seeDosage and Administration ( 2.2)].
Peripheral Edema
Insulins, including Insulin Aspart, may cause sodium retention and edema, particularly if previously poor metabolic control is improved by intensified insulin therapy.
Weight Gain
Weight gain has occurred with insulins including Insulin Aspart and has been attributed to the anabolic effects of insulin and the decrease in glucosuria.
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Insulin Aspart in the studies described below with the incidence of antibodies in other studies or to other products may be misleading.
In a 6-month study with a 6-month extension in adult subjects with type 1 diabetes, 99.8% of patients who received Insulin Aspart were positive for anti-insulin antibodies (AIA) at least once during the study, including 97.2% that were positive at baseline. A total of 92.1% of patients who received Insulin Aspart were positive for anti-drug antibodies (ADA) at least once during the study, including 64.6% that were positive at baseline.
In a phase 3 type 1 diabetes clinical trial of Insulin Aspart, initial increase in titers of antibodies to insulin, followed by a decrease to baseline values, was observed in regular human insulin and insulin aspart treatment groups with similar incidences. These antibodies did not cause deterioration in glycemic control or necessitate increases in insulin dose.
6.3 Post Marketing Experience
The following adverse reactions have been identified during post-approval use of Insulin Aspart. Because these adverse reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Medication errors have been reported in which other insulins have been accidentally substituted for Insulin Aspart.
Localized cutaneous amyloidosis at the injection site has occurred with insulin aspart. Hyperglycemia has been reported with repeated insulin injections into areas of localized cutaneous amyloidosis; hypoglycemia has been reported with a sudden change to an unaffected injection site.
7 DRUG INTERACTIONS
The table below presents clinically significant drug interactions with Insulin Aspart.
|
Drugs That May Increase the Risk of Hypoglycemia |
|
|
Drugs: |
Antidiabetic agents, ACE inhibitors, angiotensin II receptor blocking agents, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, pentoxifylline, pramlintide, salicylates, somatostatin analog (e.g., octreotide), and sulfonamide antibiotics. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when Insulin Aspart is concomitantly administered with these drugs. |
|
Drugs That May Decrease the Blood Glucose Lowering Effect of Insulin Aspart |
|
|
Drugs: |
Atypical antipsychotics (e.g., olanzapine and clozapine), corticosteroids, danazol, diuretics, estrogens, glucagon, isoniazid, niacin, oral contraceptives, phenothiazines, progestogens (e.g., in oral contraceptives), protease inhibitors, somatropin, sympathomimetic agents (e.g., albuterol, epinephrine, terbutaline), and thyroid hormones. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when Insulin Aspart is concomitantly administered with these drugs. |
|
Drugs That May Increase or Decrease the Blood Glucose Lowering Effect of Insulin Aspart |
|
|
Drugs: |
Alcohol, beta-blockers, clonidine, and lithium salts. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. Pentamidine may cause hypoglycemia, which may sometimes be followed by hyperglycemia. |
|
Intervention: |
Dose adjustment and increased frequency of glucose monitoring may be required when Insulin Aspart is concomitantly administered with these drugs. |
|
Drugs That May Blunt Signs and Symptoms of Hypoglycemia |
|
|
Drugs: |
Beta-blockers, clonidine, guanethidine and reserpine |
|
Intervention: |
Increased frequency of glucose monitoring may be required when Insulin Aspart is concomitantly administered with these drugs. |
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available information from published randomized controlled trials with insulin aspart use during the second trimester of pregnancy have not reported an association with insulin aspart and major birth defects or adverse maternal or fetal outcomes [see Data]. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
In animal reproduction studies, administration of subcutaneous insulin aspart to pregnant rats and rabbits during the period of organogenesis did not cause adverse developmental effects at exposures 8-times and equal to the human subcutaneous dose of 1 unit/kg/day, respectively.
Pre- and post-implantation losses and visceral/skeletal abnormalities were seen at higher exposures, which are considered secondary to maternal hypoglycemia. These effects were similar to those observed in rats administered regular human insulin [see Data].
In the U.S. general population, the estimated background risk of major birth defects
and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
The estimated background risk of major birth defects is 6 to 10% in women with pre-gestational
diabetes with a periconceptional HbA 1c>7% and has been reported to be as high as 20 to 25% in women with a periconceptional HbA 1c>10%. The estimated background risk of miscarriage for the indicated population is unknown.
Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Poorly controlled diabetes in pregnancy increases the maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, and delivery complications. Poorly controlled diabetes increases the fetal risk for major birth defects, stillbirth, and macrosomia related morbidity.
Data
Human Data
Published data from 5 randomized controlled trials of 441 pregnant women with diabetes mellitus treated with insulin aspart during the late 2 ndtrimester of pregnancy did not identify an association of insulin aspart with major birth defects or adverse maternal or fetal outcomes. However, these studies cannot definitely establish the absence of any risk because of methodological limitations, including a variable duration of treatment and small size of the
majority of the trials.
Animal Data
Fertility, embryo-fetal and pre- and postnatal development studies have been performed with insulin aspart and regular human insulin in rats and rabbits. In a combined fertility and embryo-fetal development study in rats, insulin aspart was administered before mating, during mating, and throughout pregnancy. Further, in a pre- and postnatal development study insulin aspart was given throughout pregnancy and during lactation to rats. In an embryo-fetal development study insulin aspart was given to female rabbits during organogenesis. The effects of insulin aspart did not differ from those observed with subcutaneous regular human insulin. Insulin aspart, like human insulin, caused pre- and post-implantation losses and visceral/skeletal abnormalities in rats at a dose of 200 units/kg/day (approximately 32 times the human subcutaneous dose of 1 unit/kg/day, based on human exposure equivalents) and in rabbits at a dose of 10 units/kg/day (approximately three times the human subcutaneous dose of 1 unit/kg/day, based on human exposure equivalents). No significant effects were observed in rats at a dose of 50 units/kg/day and in rabbits at a dose of 3 units/kg/day. These doses are approximately 8 times the human subcutaneous dose of 1 unit/kg/day for rats and equal to the human subcutaneous dose of 1 unit/kg/day for rabbits, based on human exposure equivalents. The effects are considered secondary to maternal hypoglycemia.
8.2 Lactation
Risk Summary
There are no data on the presence of Insulin Aspart in human milk, the effects on the breastfed infant, or the effect on milk production. One small published study reported that exogenous insulin, including insulin aspart, was present in human milk. However, there is insufficient information to determine the effects of insulin aspart on the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Insulin Aspart, and any potential adverse effects on the breastfed infant from Insulin Aspart, or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Insulin Aspart to improve glycemic control have been established in pediatric patients with diabetes mellitus. Use of Insulin Aspart for this indication is supported by evidence from an adequate and well-controlled study in 283 pediatric patients with type 1 diabetes mellitus aged 6 to 18 years and from studies in adults with diabetes mellitus [see Adverse Reactions (6.1), Clinical Pharmacology ( 12.3), and Clinical Studies ( 14)] .
8.5 Geriatric Use
Of the total number of patients (n=1,375) treated with Insulin Aspart in 3 controlled clinical studies, 2.6% (n=36) were 65 years of age or over. One-half of these patients had type 1 diabetes (18/1285) and the other half had type 2 diabetes (18/90). The HbA 1cresponse to Insulin Aspart, as compared to regular human insulin, did not differ by age.
10 OVERDOSAGE
Excess insulin administration may cause hypoglycemia and hypokalemia [see Warnings and Precautions ( 5.3, 5.6)] . Mild episodes of hypoglycemia usually can be treated with oral glucose. Adjustments in drug dosage, meal patterns, or exercise may be needed. More severe episodes with coma, seizure, or neurologic impairment may be treated with intramuscular/subcutaneous glucagon or concentrated intravenous glucose. Sustained carbohydrate intake and observation may be necessary because hypoglycemia may recur after apparent clinical recovery. Hypokalemia must be corrected appropriately.
11 DESCRIPTION
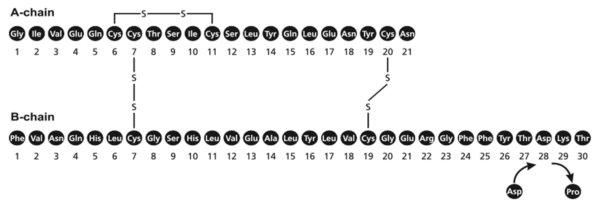
Insulin aspart is a rapid-acting human insulin analog homologous with regular human insulin with the exception of a single substitution of the amino acid proline by aspartic acid in position B28, and is produced by recombinant DNA technology utilizing Saccharomyces cerevisiae(baker's yeast). Insulin aspart has the empirical formula C 256H 381N 650 79S 6and a molecular weight of 5825.8 Da.
Insulin Aspart injection is a sterile, clear, and colorless solution for subcutaneous or intravenous use. Each mL contains 100 units of insulin aspart and the inactive ingredients: disodium hydrogen phosphate dihydrate (1.25 mg), glycerin (16.0 mg), metacresol (1.72 mg), phenol (1.50 mg), sodium chloride (0.58 mg), zinc (19.6 mcg), and Water for Injection, USP. Insulin Aspart has a pH of 7.2-7.6. Hydrochloric acid 10% and/or sodium hydroxide 10% may be added to adjust pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The primary activity of insulin, including Insulin Aspart is the regulation of glucose metabolism. Insulin and its analogs lower blood glucose by stimulating peripheral glucose uptake, especially by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulin inhibits lipolysis and proteolysis, and enhances protein synthesis.
12.2 Pharmacodynamics
Pharmacodynamics of Insulin Aspart After Subcutaneous Administration
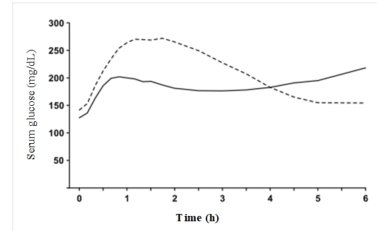
The pharmacodynamic profile of Insulin Aspart given subcutaneously in 22 patients with type 1 diabetes is shown in Figure 2. The maximum glucose-lowering effect of Insulin Aspart occurred between 1 and 3 hours after subcutaneous injection (0.15 units/kg). The duration of action for Insulin Aspart is 3 to 5 hours. The time course of action of insulin and insulin analogs such as Insulin Aspart may vary considerably in different individuals or within the same individual. The parameters of Insulin Aspart activity (time of onset, peak time and duration) as designated in Figure 2 should be considered only as general guidelines. The rate of insulin absorption and onset of activity is affected by the site of injection, exercise, and other variables [see Warnings and Precautions ( 5.3)] .
Pharmacodynamics of Insulin Aspart After Intravenous Administration
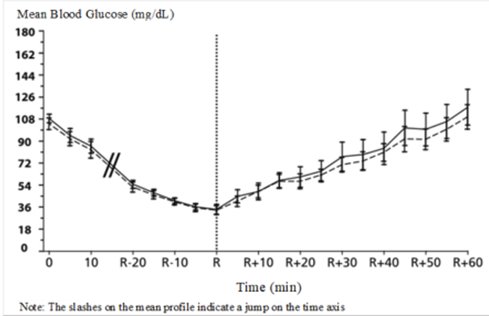
A double-blind, randomized, two-way crossover study in 16 patients with type 1 diabetes demonstrated that intravenous infusion of Insulin Aspart resulted in a blood glucose profile that was similar to that after intravenous infusion with regular human insulin. Insulin Aspart or human insulin was infused until the patient’s blood glucose decreased to 36 mg/dL, or until the patient demonstrated signs of hypoglycemia (rise in heart rate and onset of sweating), defined as the time of autonomic reaction (R) (see Figure 3).
12.3 Pharmacokinetics
Pharmacokinetics of subcutaneous administration of Insulin Aspart is presented below.
Absorption and Bioavailability
In studies in healthy volunteers (total n=107) and patients with type 1 diabetes (total n=40), the median time to maximum concentration of Insulin Aspart in these trials was 40 to 50 minutes versus 80 to 120 minutes, for regular human insulin respectively.
The relative bioavailability of Insulin Aspart (0.15 units/kg) compared to regular human insulin indicates that the two insulins are absorbed to a similar extent.
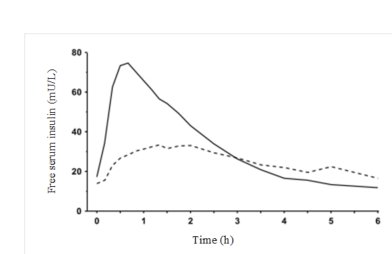
In a clinical trial in patients with type 1 diabetes, Insulin Aspart and regular human insulin, both administered subcutaneously at a dose of 0.15 units/kg body weight, reached mean maximum concentrations of 82 and 36 mU/L ,respectively.
Distribution
Insulin aspart has a low binding affinity to plasma proteins (<10%), similar to that seen with regular human insulin.
Metabolism and Elimination
In a randomized, double-blind, crossover study 17 healthy Caucasian male subjects between 18 and 40 years of age received an intravenous infusion of either Insulin Aspart or regular human insulin at 1.5 mU/kg/min for 120 minutes. The mean insulin clearance was similar for the two groups with mean values of 1.2 L/h/kg for the Insulin Aspart group and 1.2 L/h/kg for the regular human insulin group.
After subcutaneous administration in normal male volunteers (n=24), Insulin Aspart was eliminated with an average apparent half-life of 81 minutes.
Specific Populations
Pediatric Patients- The pharmacokinetic and pharmacodynamic properties of Insulin Aspart and regular human insulin were evaluated in a single dose study in 18 pediatric patients with type 1 diabetes in 2 age groups: 6-12 years, n=9 and 13-17 years (Tanner grade ≥ 2), n=9. The relative differences in pharmacokinetics and pharmacodynamics in the pediatric patients with type 1 diabetes in both age groups between Insulin Aspart and regular human insulin were similar to those in healthy adult subjects and adults with type 1 diabetes.
Geriatric Patients: The pharmacokinetic and pharmacodynamic properties of Insulin Aspart and regular human insulin were investigated in a single dose study in 18 subjects with type 2 diabetes who were ≥ 65 years of age. The relative differences in pharmacokinetics and pharmacodynamics in geriatric patients with type 2 diabetes between Insulin Aspart and regular human insulin were similar to those in younger adults.
Male and Female Patients: In healthy volunteers given a single subcutaneous dose of Insulin Aspart 0.06 units/kg, no difference in insulin aspart levels was seen between males and females based on comparison of AUC (0-10h)or C max.
Obese Patients: A single subcutaneous dose of 0.1 units/kg Insulin Aspart was administered in a study of 23 patients with type 1 diabetes and a wide range of body mass index (BMI, 22-39 kg/m 2). The pharmacokinetic parameters, AUC and C max, of Insulin Aspart were generally unaffected by BMI in the different groups – BMI 19-23 kg/m 2(n=4); BMI 23-27 kg/m 2(n=7); BMI 27-32 kg/m 2(n=6) and BMI >32 kg/m 2(n=6). Clearance of Insulin Aspart was reduced by 28% in patients with BMI >32 kg/m 2compared to patients with BMI <23 kg/m 2.
Patients with Renal Impairment: A single subcutaneous dose of 0.08 units/kg Insulin Aspart was administered in a study to subjects with either normal renal function (n=6) creatinine clearance (CLcr) (> 80 ml/min) or mild (n=7; CLcr = 50-80 ml/min), moderate (n=3; CLcr = 30-50 ml/min) or severe (but not requiring hemodialysis) (n=2; CLcr = <30 ml/min) renal impairment. In this study, there was no apparent effect of creatinine clearance values on AUC and C maxof Insulin Aspart.
Patients with Hepatic Impairment: A single subcutaneous dose of 0.06 units/kg Insulin Aspart was administered in an open-label, single-dose study of 24 subjects (n=6/group) with different degree of hepatic impairment (mild, moderate and severe) having Child-Pugh Scores ranging from 0 (healthy volunteers) to 12 (severe hepatic impairment). In this study, there was no correlation between the degree of hepatic impairment and any Insulin Aspart pharmacokinetic parameter.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Standard 2-year carcinogenicity studies in animals have not been performed to evaluate the carcinogenic potential of Insulin Aspart. In 52-week studies, Sprague-Dawley rats were dosed subcutaneously with Insulin Aspart at 10, 50, and 200 units/kg/day (approximately 2, 8, and 32 times the human subcutaneous dose of 1.0 units/kg/day, based on units/body surface area, respectively). At a dose of 200 units/kg/day, Insulin Aspart increased the incidence of mammary gland tumors in females when compared to untreated controls. The relevance of these findings to humans is unknown.
Insulin Aspart was not genotoxic in the following tests: Ames test, mouse lymphoma cell forward gene mutation test, human peripheral blood lymphocyte chromosome aberration test, in vivomicronucleus test in mice, and in ex vivoUDS test in rat liver hepatocytes.
In fertility studies in male and female rats, at subcutaneous doses up to 200 units/kg/day (approximately 32 times the human subcutaneous dose, based on units/body surface area), no direct adverse effects on male and female fertility, or general reproductive performance of animals was observed.
14 CLINICAL STUDIES
14.1 Overview of Clinical Studies
The safety and effectiveness of subcutaneous Insulin Aspart were compared to regular human insulin in 596 type 1 diabetes adult, 187 pediatric type 1 diabetes, and 91 adult type 2 diabetes patients using NPH as basal insulin (see Tables 3, 4, 5). The reduction in glycated hemoglobin (HbA 1c) was similar to regular human insulin.
The safety and effectiveness of Insulin Aspart administered by continuous subcutaneous insulin infusion (CSII) by external pump were compared to buffered regular human insulin (administered by CSII), to lispro (administered by CSII) and compared to Insulin Aspart injections and NPH injection. Overall, the reduction in HbA 1cwas similar to the comparator.
14.2 Clinical Studies in Adult and Pediatric Patients with Type 1 Diabetes with Subcutaneous Injections
Type 1 Diabetes – Adults (see Table 3)
Two 24-week, open‑label, active-controlled studies were conducted to compare the safety and efficacy of Insulin Aspart to regular human insulin injection in adult patients with type 1 diabetes. Because the two study designs and results were similar, data are shown for only one study (see Table 3).
The mean age of the trial population was 39 years and mean duration of diabetes was 15.7 years. Fifty-one percent were male. Ninety-four percent were Caucasian, 2% were Black and 4% were Other. The mean BMI was approximately 25.6 kg/m 2.
Insulin Aspart was administered by subcutaneous injection immediately prior to meals and regular human insulin was administered by subcutaneous injection 30 minutes before meals. NPH insulin was administered as the basal insulin in either single or divided daily doses. Changes in HbA 1cwere comparable for the two treatment regimens in this study (Table 3).
Table 3. Type 1 Diabetes Mellitus – Adult (Insulin Aspart plus NPH insulin vs. regular human insulin plus NPH insulin)
|
Insulin Aspart + NPH (n=596) |
Regular Human Insulin+ NPH (n=286) |
|
|
Baseline HbA 1c(%)* |
7.9 ±1.1 |
8.0 ± 1.2 |
|
Change from Baseline HbA 1c(%) |
-0.1 ± 0.8 |
0.0 ± 0.8 |
|
Treatment Difference in HbA 1c, Mean (95% confidence interval) |
-0.2 (-0.3, -0.1) |
|
*Values are Mean ± SD
Type 1 Diabetes – Pediatric ( seeTable 4)
The efficacy of Insulin Aspart to improve glycemic control in pediatric patients with type 1 diabetes mellitus is based on an adequate and well-controlled trial of regular human insulin in pediatric patients with type 1 diabetes mellitus (Table 4). This 24-week, parallel-group study of pediatric patients with type 1 diabetes (n=283), aged 6 to 18 years, compared two subcutaneous multiple-dose treatment regimens: Insulin Aspart (n=187) or regular human insulin (n=96). NPH insulin was administered as the basal insulin. Similar effects on HbA 1cwere observed in both treatment groups (Table 4).
Subcutaneous administration of Insulin Aspart and regular human insulin have also been compared in pediatric patients with type 1 diabetes (n=26) aged 2 to 6 years with similar effects on HbA 1c.
Table 4. Pediatric Subcutaneous Administration of Insulin Aspart in Type 1 Diabetes(24 weeks; n=283)
|
Insulin Aspart + NPH (n=187) |
Regular Human Insulin+ NPH (n=96) |
|
|
Baseline HbA 1c(%)* |
8.3 ± 1.2 |
8.3 ± 1.3 |
|
Change from Baseline HbA 1c(%) |
0.1± 1.0 |
0.1± 1.1 |
|
Treatment Difference in HbA 1c,Mean (95% confidence interval) |
-0.2 (-0.5, 0.1) |
|
*Values are Mean ± SD
14.3 Clinical Studies in Adults with Type 2 Diabetes with Subcutaneous Injections
Type 2 Diabetes - Adults ( seeTable 5)
One six-month, open-label, active-controlled study was conducted to compare the safety and efficacy of Insulin Aspart to regular human insulin in patients with type 2 diabetes (Table 5).
The mean age of the trial population was 56.6 years and mean duration of diabetes was 12.7 years. Sixty-three percent were male. Seventy-six percent were Caucasian, 9% were Black and 15% were Other. The mean BMI was approximately 29.7 kg/m 2.
Insulin Aspart was administered by subcutaneous injection immediately prior to meals and regular human insulin was administered by subcutaneous injection 30 minutes before meals. NPH insulin was administered as the basal insulin in either single or divided daily doses. Changes in HbA 1cwere comparable for the two treatment regimens.
Table 5. Subcutaneous Insulin Aspart Administration in Type 2 Diabetes(6 months; n=176)
|
Insulin Aspart + NPH (n=90) |
Regular Human Insulin + NPH (n=86) |
|
|
Baseline HbA 1c(%)* |
8.1 ± 1.2 |
7.8 ± 1.1 |
|
Change from Baseline HbA 1c(%) |
-0.3 ± 1.0 |
-0.1 ± 0.8 |
|
Treatment Difference in HbA 1c,Mean (95% confidence interval) |
- 0.1 (-0.4, 0.1) |
|
*Values are Mean ± SD
14.4 Clinical Studies in Adults and Pediatrics with Type 1 Diabetes Using Continuous Subcutaneous Insulin Infusion (CSII) by External Pump
Type 1 Diabetes – Adult ( seeTable 6)
Two open-label, parallel design studies (6 weeks [n=29] and 16 weeks [n=118]) compared Insulin Aspart to buffered regular human insulin (Velosulin) in adults with type 1 diabetes receiving a subcutaneous infusion with an external insulin pump.
The mean age of the trial population was 42.3 years. Thirty-nine percent were male. Ninety-eight percent were Caucasian and 2% were Black.
The two treatment regimens had comparable changes in HbA 1c.
Table 6. Adult Insulin Pump Study in Type 1 Diabetes(16 weeks; n=118)
|
Insulin Aspart (n=59) |
Buffered human insulin (n=59) |
|
|
Baseline HbA 1c(%)* |
7.3 ± 0.7 |
7.5 ± 0.8 |
|
Change from Baseline HbA 1c(%) |
0.0 ± 0.5 |
0.2 ± 0.6 |
|
Treatment Difference in HbA 1c,Mean (95% confidence interval) |
0.2 (-0.1, 0.4) |
|
*Values are Mean ± SD
Type 1 Diabetes – Pediatric ( seeTable 7)
A randomized, 16-week, open-label, parallel design study of pediatric patients with type 1 diabetes (n=298) aged 4-18 years compared two subcutaneous infusion regimens administered via an external insulin pump: Insulin Aspart (n=198) or insulin lispro (n=100). These two treatments resulted in comparable changes from baseline in HbA 1c(see Table 7).
Table 7. Pediatric Insulin Pump Study in Type 1 Diabetes(16 weeks; n=298)
|
Insulin Aspart (n=198) |
Lispro (n=100) |
|
|
Baseline HbA 1c(%)* |
8.0 ± 0.9 |
8.2 ± 0.8 |
|
Change from Baseline HbA 1c(%) |
-0.1 ± 0.8 |
-0.1 ± 0.7 |
|
Treatment Difference in HbA 1c,Mean (95% confidence interval) |
-0.1 (-0.3, 0.1) |
|
*Values are Mean ± SD
14.5 Clinical Studies in Adults with Type 2 Diabetes Using Continuous Subcutaneous Insulin Infusion (CSII) by External Pump
Type 2 Diabetes – Adults ( seeTable 8)
An open-label, 16-week parallel design trial compared pre-prandial Insulin Aspart injection in conjunction with NPH injections to Insulin Aspart administered by continuous subcutaneous infusion in 127 adults with type 2 diabetes.
The mean age of the trial population was 55.1 years. Sixty-four percent were male. Eighty percent were Caucasian, 12% were Black and 8% were Other. The mean BMI was approximately 32.2 kg/m 2.
The two treatment groups had similar reductions in HbA 1c(Table 8).
Table 8. Pump Therapy in Type 2 Diabetes(16 weeks; n=127)
|
Insulin Aspart pump (n=66) |
Insulin Aspart + NPH (n=61) |
|
|
Baseline HbA 1c(%)* |
8.2 ± 1.4 |
8.0 ± 1.1 |
|
Change from Baseline HbA 1c(%) |
-0.6 ± 1.1 |
-0.5 ± 0.9 |
|
Treatment Difference in HbA 1c,Mean (95% confidence interval) |
0.1 (-0.3, 0.4) |
|
*Values are Mean ± SD
16 HOW SUPPLIED/STORAGE AND HANDLING
Insulin Aspart injection 100 units/mL (U-100) is available as a clear and colorless solution in:
NDC: 70518-2823-00
PACKAGING: 1 CARTON, 10 mL VIAL, GLASS TYPE 0
*Insulin Aspart PenFill cartridges are designed for use with compatible insulin delivery devices with NovoFine disposable needles. FlexPen can be used with NovoFine or NovoTwist disposable needles.
The Insulin Aspart FlexPen dials in 1-unit increments.
16.2 Recommended Storage
Dispense in the original sealed carton with the enclosed Instructions for Use. Store unused Insulin Aspart in a refrigerator between 2°C to 8°C (36°F to 46°F). Do not freeze Insulin Aspart and do not use Insulin Aspart if it has been frozen. Do not expose Insulin Aspart to excessive heat or light. Do not withdraw Insulin Aspart into a syringe and store for later use.
Always remove and discard the needle after each injection from the Insulin Aspart FlexPen and store without a needle attached.
The storage conditions are summarized in the following table:
Not in-use (unopened) Room Temperature (up to 30°C [86°F]) : 28 days
Not in-use (unopened) Refrigerated (2°C to 8°C [36°F to 46°F]): until expiration date
In-use (opened) Room Temperature (up to 30°C [86°F]): 28 days (refrigerated/room temperature)
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Never Share an Insulin Aspart FlexPen, PenFill Cartridge or PenFill Cartridge Device between Patients
Advise patients that they must never share Insulin Aspart FlexPen, PenFill cartridge or PenFill cartridge devices with another person even if the needle is changed, because doing so carries a risk for transmission of blood-borne pathogens. Advise patients using Insulin Aspart vials not to share needles or syringes with another person. Sharing poses a risk for transmission of blood-borne pathogens [seeWarnings and Precautions ( 5.1)] .
Hyperglycemia or Hypoglycemia
Inform patients that hypoglycemia is the most common adverse reaction with insulin. Instruct patients on self-management procedures including glucose monitoring, proper injection technique, and management of hypoglycemia and hyperglycemia, especially at initiation of Insulin Aspart therapy. Instruct patients on handling of special situations such as intercurrent conditions (illness, stress, or emotional disturbances), an inadequate or skipped insulin dose, inadvertent administration of an increased insulin dose, inadequate food intake, and skipped meals. Instruct patients on the management of hypoglycemia [see Warnings and Precautions ( 5.3)] .
Inform patients that their ability to concentrate and react may be impaired as a result of hypoglycemia. Advise patients who have frequent hypoglycemia or reduced or absent warning signs of hypoglycemia to use caution when driving or operating machinery.
Advise patients that changes in insulin regimen can predispose to hyperglycemia or hypoglycemia and that changes in insulin regimen should be made under close medical supervision [see Warnings and Precautions ( 5.2)] .
Hypoglycemia with Medication Errors
Instruct patients to always check the insulin label before each injection to avoid mix-ups between insulin products [seeWarnings and Precautions ( 5.3)] .
Hypersensitivity Reactions
Advise patients that hypersensitivity reactions have occurred with Insulin Aspart. Inform patients of the symptoms of hypersensitivity reactions [seeWarnings and Precautions ( 5.4)] .
Patients Using Continuous Subcutaneous Insulin Pumps
- Train patients in both intensive insulin therapy with multiple injections and in the function of their pump and pump accessories.
- This Insulin Aspart product can be used with continuous subcutaneous insulin infusion pumps labeled for use with NOVOLOG (insulin aspart) - refer to the insulin pump user manual to see if NOVOLOG can be used. See recommended infusion sets in the insulin pump user manual.
- Instruct patients to replace insulin in the reservoir at least every 7 days or according to the user manual, whichever is shorter, infusion sets and infusion set insertion sites should be changed in according to the manufacturer’s user manual. By following this schedule, patients avoid insulin degredation, infusion set occlusion, and loss of the insulin preservative.
- Instruct patients to discard insulin exposed to temperatures higher than 37°C (98.6°F).
- Instruct patients to inform physician and select a new site for infusion if infusion site becomes erythematous, pruritic, or thickened.
- Instruct patients of the risk of rapid hyperglycemia and ketosis due to pump malfunction, infusion set occlusion, leakage, disconnection or kinking, and degraded insulin. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician [see Warnings and Precautions ( 5) and How Supplied/Storage and Handling ( 16.2)] .
- Instruct patients of the risk of hypoglycemia from pump malfunction. If these problems cannot be promptly corrected, instruct patients to resume therapy with subcutaneous insulin injection and contact their physician [see Warnings and Precautions ( 5) and How Supplied/Storage and Handling ( 16.2)] .
Novo Nordisk ®, NovoLog ®, PenFill ®, Novolin ®, FlexPen ®, NovoFine ®, and NovoTwist ®are registered trademarks of Novo Nordisk A/S.
Patent Information: http://novonordisk-us.com/products/product-patents.html
© 2023 Novo Nordisk
1-800-727-6500
Repackaged By / Distributed By: RemedyRepack Inc.
625 Kolter Drive, Indiana, PA 15701
(724) 465-8762
PATIENT INFORMATION
Insulin Aspart[ in∙suh∙luhn a∙sprt]
injection, for subcutaneous or intravenous use
This product is NovoLog (insulin aspart).
Do not share your Insulin Aspart FlexPen, PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
What is Insulin Aspart?
- Insulin Aspart is a man-made insulin that is used to control high blood sugar in adults and children with diabetes mellitus.
Who should not take Insulin Aspart?
Do not take Insulin Aspart if you:
- are having an episode of low blood sugar (hypoglycemia).
- have an allergy to Insulin Aspart or any of the ingredients in Insulin Aspart.
Before taking Insulin Aspart, tell your healthcare provider about all your medical conditions including, if you are:
- pregnant, planning to become pregnant, or are breastfeeding.
- taking new prescription or over-the-counter medicines, vitamins, or herbal supplements.
Before you start taking Insulin Aspart, talk to your healthcare provider about low blood sugar and how to manage it.
How should I take Insulin Aspart?
- Read the Instructions for Usethat come with your Insulin Aspart.
- Take Insulin Aspart exactly as your healthcare provider tells you to.
- Insulin Aspart starts acting fast.You should eat a meal within 5 to 10 minutes after you take your dose of Insulin Aspart.
- Know the type and strength of insulin you take. Do notchange the type of insulin you take unless your healthcare provider tells you to. The amount of insulin and the best time for you to take your insulin may need to change if you take different types of insulin.
- Check your blood sugar levels.Ask your healthcare provider what your blood sugars should be and when you should check your blood sugar levels.
- Do not reuse or share your needles with other people.You may give other people a serious infection or get a serious infection from them.
- Insulin Aspart can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs) or upper arms, or by continuous infusion under the skin (subcutaneously) through an insulin pump into an area of your body recommended in the instructions that come with your insulin pump.
- Change (rotate) your injection sites within the area you choose with each doseto reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites.
- Do notuse the exact same spot for each injection.
- Do notinject where the skin has pits, is thickened, or has lumps.
- Do notinject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
What should I avoid while taking Insulin Aspart?
While taking Insulin Aspart do not:
- Drive or operate heavy machinery, until you know how Insulin Aspart affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of Insulin Aspart?
Insulin Aspart may cause serious side effects that can lead to death, including:
Low blood sugar (hypoglycemia).Signs and symptoms that may indicate low blood sugar include:
- dizziness or light-headedness
- blurred vision
- anxiety, irritability, or mood changes
- sweating
- slurred speech
- hunger
- confusion
- shakiness
- headache
- fast heart beat
Your insulin dose may need to change because of:
- change in level of physical activity or exercise
- increased stress
- change in diet
- weight gain or loss
- illness
Other common side effects of Insulin Aspart may include:
- low potassium in your blood (hypokalemia), reactions at the injection site, itching, rash, serious allergic reactions (whole body reactions), skin thickening or pits at the injection site (lipodystrophy), weight gain, and swelling of your hands and feet.
Get emergency medical help if you have:
- trouble breathing, shortness of breath, fast heartbeat, swelling of your face, tongue, or throat, sweating, extreme drowsiness, dizziness, confusion.
These are not all the possible side effects of Insulin Aspart. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Insulin Aspart.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about Insulin Aspart that is written for health professionals. Do not use Insulin Aspart for a condition for which it was not prescribed. Do not give Insulin Aspart to other people, even if they have the same symptoms that you have. It may harm them.
What are the ingredients in Insulin Aspart?
Active Ingredient:insulin aspart
Inactive Ingredients:disodium hydrogen phosphate dihydrate, glycerin, metacresol, phenol, sodium chloride, zinc, and Water for Injection, USP. Hydrochloric acid 10% and/or sodium hydroxide 10% may be added to adjust pH.
Manufactured by:Novo Nordisk Inc., Plainsboro, NJ 08536, U.S. License Number 1261
For more information, call 1-800-727-6500.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 02/2023
INSTRUCTIONS FOR USE
Insulin Aspart [in∙suh∙luhn a∙sprt]
injection, for subcutaneous or intravenous use
10 mL multiple-dose vial (100 units/mL, U-100)
This product is NovoLog (insulin aspart).
Read this Instructions for Use before you start taking Insulin Aspart and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Supplies you will need to give your Insulin Aspart injection:
- 10 mL Insulin Aspart vial
- insulin syringe and needle
- alcohol swabs
Preparing your Insulin Aspart dose:
- Wash your hands with soap and water.
- Before you start to prepare your injection, check the Insulin Aspart label to make sure that you are taking the right type of insulin. This is especially important if you use more than 1 type of insulin.
- Insulin Aspart should look clear and colorless. Do notuse Insulin Aspart if it is thick, cloudy, or is colored.
- Do notuse Insulin Aspart past the expiration date printed on the label.
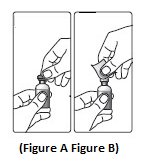
Step 1:Pull off the tamper resistant cap (See Figure A).
Step 2:Wipe the rubber stopper with an alcohol swab (See Figure B).
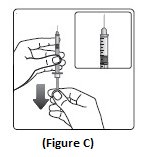
Step 3:Hold the syringe with the needle pointing up. Pull down on the plunger until the black tip reaches the line for the number of units for your prescribed dose (See Figure C).
Step 4:Push the needle through the rubber stopper of the Insulin Aspart vial (See Figure D).
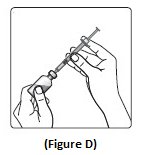
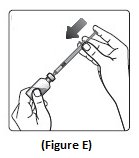
Step 5:Push the plunger all the way in. This puts air into the Insulin Aspart vial (See Figure E).
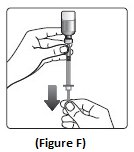
Step 6:Turn the Insulin Aspart vial and syringe upside down and slowly pull the plunger down until the black tip is a few units past the line for your dose (See Figure F).
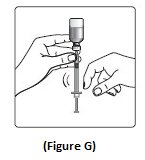
- If there are air bubbles, tap the syringe gently a few times to let any air bubbles rise to the top (See Figure G).
Step 7:Slowly push the plunger up until the black tip reaches the line for your Insulin Aspart dose (See Figure H).
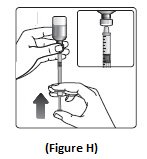
Step 8:Check the syringe to make sure you have the right dose of Insulin Aspart.
Step 9:Pull the syringe out of the vial’s rubber stopper (See Figure I).
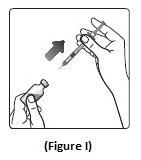
- Giving your Injection:
- Inject your Insulin Aspart exactly as your healthcare provider has shown you. Your healthcare provider should tell you if you need to pinch the skin before injecting.
- Insulin Aspart can be injected under the skin (subcutaneously) of your stomach area, buttocks, upper legs or upper arms, infused in an insulin pump (continuous subcutaneous infusion into an area of your body recommended in the instructions that come with your insulin pump), or given through a needle in your arm (intravenously) by your healthcare provider.
- If you inject Insulin Aspart, change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do notuse the same injection site for each injection. Do notinject where the skin has pits, is thickened, or has lumps. Do notinject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- If you use Insulin Aspart in an insulin pump, you should change your infusion set and insertion site according to the manufacturer’s user manual. This Insulin Aspart product can be used with the continuous subcutaneous insulin infusion pumps labeled for use with NovoLog (insulin aspart) - refer to the insulin pump user manual to see if NovoLog can be used. Insulin Aspart should be given into an area of your body recommended in the instructions that come with your insulin pump. Change (rotate) your insertion sites within the area you choose for each insertion to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the insertion sites. Do not insert into the exact same spot for each insertion. Do not insert where the skin has pits, is thickened, or has lumps. Do not insert where the skin is tender, bruised, scaly or hard, or into scars or damaged skin. The insulin in the reservoir should be changed at least every 7 days or according to the pump user manual, whichever is shorter, even if you have not used all of the insulin.
- If you use Insulin Aspart in an insulin pump, see your insulin pump manual for instructions or talk to your healthcare provider.
- NPH insulin is the only type of insulin that can be mixed with Insulin Aspart. Do notmix Insulin Aspart with any other type of insulin.
- Insulin Aspart should onlybe mixed with NPH insulin if it is going to be injected right away under your skin (subcutaneously).
- Insulin Aspart should be drawn up into the syringe beforeyou draw up your NPH insulin.
- Talk to your healthcare provider if you are not sure about the right way to mix Insulin Aspart and NPH insulin.
Step 10: Choose your injection site (stomach area, buttocks, upper legs or upper arms) and wipe the skin with an alcohol swab. Let the injection site dry before you inject your dose (See Figure J).
(Figure J)
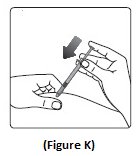
Step 11:Insert the needle into your skin. Push down on the plunger to inject your dose (See Figure K). The needle should remain in the skin for at least 6 seconds to make sure you have injected all the insulin.
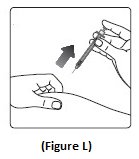
Step 12:Pull the needle out of your skin. After that, you may see a drop of Insulin Aspart at the needle tip. This is normal and does not affect the dose you just received (See Figure L).
- If you see blood after you take the needle out of your skin, press the injection site lightly with a
- piece of gauze or an alcohol swab. Do notrub the area.
After your injection:
- Do notrecap the needle. Recapping the needle can lead to a needle stick injury.
- Put the empty insulin vials, used needles and syringes in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes and needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
How should I store Insulin Aspart?
- Do notfreeze Insulin Aspart. Do notuse Insulin Aspart if it has been frozen.
- Keep Insulin Aspart away from heat or light.
- All unopened vials:
- Store unopened Insulin Aspart vials in the refrigerator at 36 °F to 46 °F (2 °C to 8 °C).
- Unopened vials may be used until the expiration date printed on the label, if they have been stored in the refrigerator.
- Unopened vials should be thrown away after 28 days, if they are stored at room temperature.
- After vials have been opened:
- Opened Insulin Aspart vials can be stored in the refrigerator at 36 °F to 46 °F (2 °C to 8 °C) or at room temperature below 86 °F (30 °C).
- Throw away all opened Insulin Aspart vials after 28 days, even if they still have insulin left in them.
- If using Insulin Aspart in a pump, throw away all opened Insulin Aspart vials after 19 days.
General information about the safe and effective use of Insulin Aspart
- Always use a new syringe and needle for each injection.
- Do not share syringes or needles.
- Keep Insulin Aspart vials, syringes, and needles out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
INSTRUCTIONS FOR USE
Insulin Aspart [in∙suh∙luhn a∙sprt]
injection, for subcutaneous or intravenous use
PenFill ®3 mL cartridge 100 units/mL (U-100)
This product is NovoLog (insulin aspart).
- Do not share your PenFill cartridge or PenFill cartridge compatible insulin delivery device with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
- Your healthcare provider should show you or your caregiver how to inject Insulin Aspart the right way before you inject it for the first time.
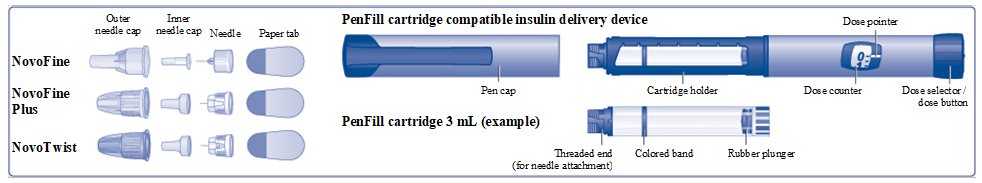
- Insulin Aspart PenFill cartridge 100 units/mL is a prefilled, single-patient-use cartridgecontaining 300 units of Insulin Aspart.
- After you insert the PenFill cartridge in your device, you can use it for multiple injections. Read the instruction manualthat comes with your insulin delivery device for complete instructions on how to use the PenFill cartridge with the device.
- People who are blind or have vision problems should not use this PenFill cartridge without help from a person trained to use the PenFill cartridge with the device.
- If using a newInsulin Aspart PenFill cartridge, start with Step 1.
- If the Insulin Aspart PenFill cartridge has already been used, start with Step 2.
Supplies you will need to give your Insulin Aspart injection:
- Insulin Aspart PenFill cartridge
- Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device
- 1 new NovoFine, NovoFine Plus, or NovoTwist needle
- Alcohol swabs
- Adhesive bandage
- Cotton gauze
- A sharps container for throwing away used PenFill cartridges and needles. See “After your injection” at the end of these instructions.
(Figure A)
How to use the Insulin Aspart PenFill cartridge
- Wash your hands with soap and water.
- Before you start to prepare your injection, check theInsulin Aspart PenFill cartridge labelto make sure that it contains the insulin you need. This is especially important if you take more than 1 type of insulin.
- The tamper-resistant foil should be in place before the first use. If the foil has been broken or removed before your first use of the cartridge, do notuse it. Call Novo Nordisk at 1-800-727-6500.
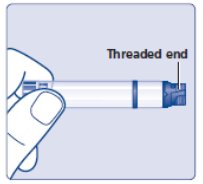
- Carefully look at the cartridge and the insulin inside it. Check that the Insulin Aspart cartridge:
- is not damaged, for example cracked or leaking
- is not loose on the threaded end
- Insulin Aspart should look clear and colorless. Do notuse Insulin Aspart if it is cloudy or colored or if the threaded end is loose (See Figure B).
(Figure B)
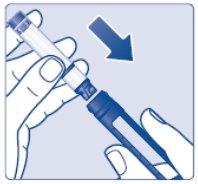
Step 1:
- Insert a 3 mL cartridge with the threaded end first into your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device (See Figure C).
- If you drop your device, check the insulin cartridge for damage such as cracks or leaking. If your cartridge is damaged, throw it away and use a new one.
(Figure C)
Prepare your device with a new needle
Step 2:
- Take a new needle,and tear off the paper tab. Always use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not attach a new needleto your device until you are ready to give your injection. Do not reuse or share your needles with other people. You may give others a serious infection, or get a serious infection from them.
- Be careful not to bend or damage the needle before you use it.
- Push the needle straight onto the device. Turn the needle clockwise until it is on tight (See Figure D).
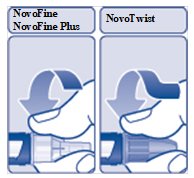
(Figure D)
Step 3:
- Pull off the outer needle cap(See Figure E) . Do notthrow it away. You will need it after the injection to safely remove the needle.
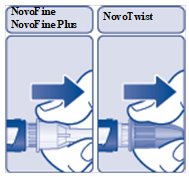
(Figure E)
Step 4:
- Pull off the inner needle capand throw it away (See Figure F). Do nottry to put the inner needle cap back on the needle.
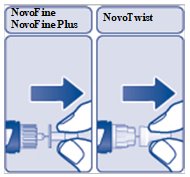
(Figure F)
A drop of insulin may appear at the needle tip. This is normal, but you must still check the insulin flow.
- Check the insulin flow
Step 5:
- Small amounts of air may collect in the cartridge during normal use. You must do an airshot before each injection to avoid injecting air and to make sure you receive the prescribed dose of your medicine.
- Do the airshot as described in the instruction manual that comes with your device.
- Keep testing your Novo Nordisk 3 mL PenFill cartridge compatible insulin delivery device until you see insulin at the needle tip. If you still do not see a drop of insulin after 6 times, change the needle and repeat this step. This makes sure that any air bubbles are removed and that insulin is getting through the needle (See Figure G).
(Figure G)
Select your dose
Step 6:
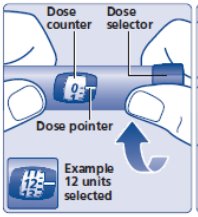
- Check to make sure that the dose counter is set to 0.
- Turn the dose selector clockwise to select the dose you needto inject (See Figure H). The pointer should line up with your dose. When turning the dose selector, be careful not to press the dose button as insulin will come out. You will hear a click for every single unit dialed. Do notset the dose by counting the number of clicks you hear because you may get an incorrect dose.
- Refer to your insulin delivery device manual if necessary.
(Figure H)
Inject your dose
Step 7:
- Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting.
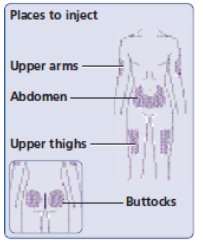
- Insulin Aspart can be injected under the skin (subcutaneously) of your stomach area (abdomen), buttocks, upper legs (thighs), or upper arms (See Figure I).
- (Figure I)
- Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same injection site for each injection. Do not inject where the skin has pits, is thickened, or has lumps. Do not inject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
- Clean your injection site with an alcohol swab. Let your skin dry. Do not touch this area again before injecting.
- Insert the needle into your skin. Press and hold down the dose button until the dose counter shows “0”. Continue to keep the dose button pressed and keep the needle in your skin and slowly count to 6 (see Figure J).
- Remove the needle from your skin.
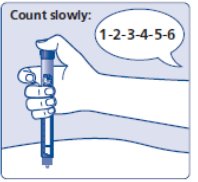
(Figure J)
You may see a drop of Insulin Aspart at the needle tip after injecting. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with a cotton gauze and cover with an adhesive bandage, if necessary. Do not rub the area.
After your injection
Step 8:
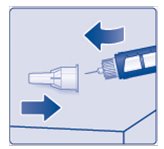
- Lay your outer needle cap on a flat surface. Carefully, lead the needle tip into the outer needle cap without touching the needle (See Figure K) and push the outer needle cap completely on.
(Figure K)
- Hold the black cartridge holder on the insulin delivery device and unscrew the needle counterclockwise (See Figure L).
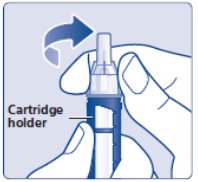
(Figure L)
- Throw away (dispose of) the needle in an FDA-cleared sharps container as your healthcare professional has instructed you.
- Put your empty Insulin Aspart PenFill cartridge and used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and PenFill cartridges in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use, and
- leak-resistant and properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. Do not reuse or share your needles or syringes with other people. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Step 9:
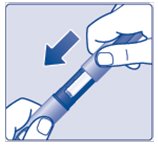
- Keep the 3 mL PenFill cartridge in the device. Do notstore your device with a needle attached. This will prevent infection or leakage of insulin and will make sure that you receive the right dose of Insulin Aspart.
- Put the pen cap onyour device after each use to protect the insulin from light (See Figure M).
(Figure M)
How should I store my Insulin Aspart PenFill cartridge?
- Do notfreeze Insulin Aspart. Do notuse Insulin Aspart if it has been frozen.
- Keep Insulin Aspart away from heat or light.
- Store the Insulin Aspart PenFill cartridge without the needle attached.
Before use:
- Store unused Insulin Aspart PenFill cartridges in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused PenFill cartridges may be used until the expiration date printed on the label, if kept in the refrigerator.
- If Insulin Aspart is stored mistakenly outside of refrigeration between 47°F (9°C) to 86°F (30°C) prior to first use, it should be used within 28 days or thrown away.
PenFill cartridges in use:
- Store the PenFill cartridge you are currently using in the insulin delivery device at room temperature below 86°F (30°C) for up to 28 days. Do not refrigerate.
- The Insulin Aspart PenFill cartridge you are using should be thrown away after 28 days, even if it still has insulin left in it.
General Information about the safe and effective use of Insulin Aspart.
- Keep Insulin Aspart PenFill cartridges and needles out of the reach of children.
- Do notshare Insulin Aspart PenFill cartridges or needles with other people. You may give other people a serious infection, or get a serious infection from them.
- Always carry extra insulin of the same type(s) you use in case of loss or damage.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
© 2023 Novo Nordisk
Manufactured by:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536
U.S. License Number 1261
Revised: 02/2023
INSTRUCTIONS FOR USE
Insulin Aspart [in∙suh∙luhn a∙sprt]
injection, for subcutaneous or intravenous use
3 mL FlexPen ®prefilled pen (100 units/mL, U-100)
This product is NovoLog (insulin aspart).
Introduction
Please read the following instructions carefully before using your Insulin Aspart FlexPen.
Do not share your Insulin Aspart FlexPen with other people, even if the needle has been changed. You may give other people a serious infection, or get a serious infection from them.
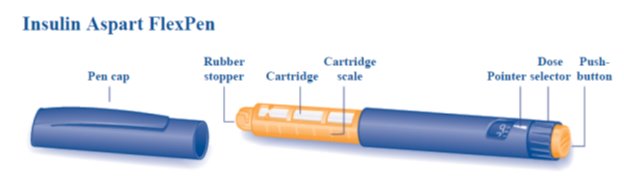
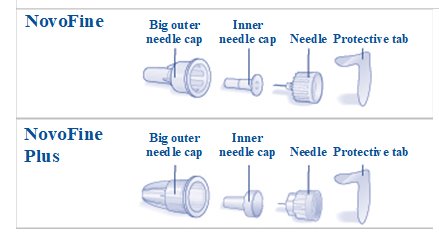
Insulin Aspart FlexPen is a disposable, single-patient-use, dial-a-dose insulin pen. You can select doses from 1 to 60 units in increments of 1 unit. Insulin Aspart FlexPen is designed to be used with NovoFine, NovoFine Plus or NovoTwist needles.
- Δ People who are blind or have vision problems should not use this Pen without help from a person trained to use the Pen.
Getting ready
Make sure you have the following items:
- Insulin Aspart FlexPen
- New NovoFine, NovoFine Plus or NovoTwist needle
- Alcohol swabs
Preparing your Insulin Aspart FlexPen
Wash your hands with soap and water. Before you start to prepare your injection, check the label to make sure that you are taking the right type of insulin. This is especially important if you take more than 1 type of insulin. Insulin Aspart should look clear and colorless. Do not use your Insulin Aspart FlexPen if the liquid contains particles or is colored.
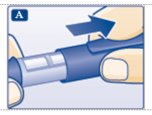
A. Pull off the pen cap (see diagram A).
Wipe the rubber stopper with an alcohol swab.
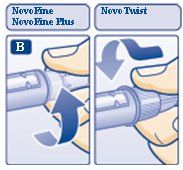
B. Attaching the needle
Remove the protective tab from a disposable needle.
Screw the needle tightly onto your FlexPen. It is important that the needle is put on straight (see diagram B).
Never place a disposable needle on your Insulin Aspart FlexPen until you are ready to take your injection.
C.Pull off the big outer needle cap (see diagram C).
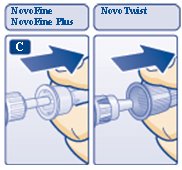
D.Pull off the inner needle cap and throw it away (dispose of it) (see diagram D).
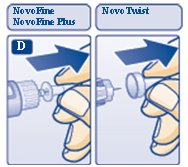
- ΔAlways use a new needle for each injection to make sure the needle is free of germs (sterile) and to prevent blocked needles. Do not reuse or share your needles with other people. You may give other people a serious infection, or get a serious infection from them.
- ΔBe careful not to bend or damage the needle before use.
- ΔTo reduce the risk of unexpected needle sticks, never put the inner needle cap back on the needle.
Giving the airshot before each injection
Before each injection small amounts of air may collect in the cartridge during normal use. To avoid injecting air and to ensure proper dosing:
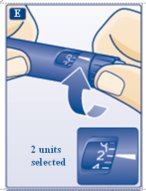
E.Turn the dose selector to select 2 units (see diagram E).
F.Hold your Insulin Aspart FlexPen with the needle pointing up. Tap the cartridge gently with your finger a few times to make any air bubbles collect at the top of the cartridge (see diagram F).
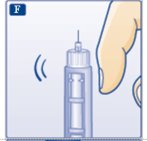
G.Keep the needle pointing upwards, press the push-button all the way in (see diagram G). The dose selector returns to 0.
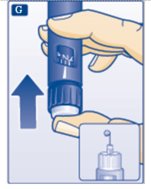
A drop of insulin should appear at the needle tip. If not, change the needle and repeat the procedure no more than 6 times.
If you do not see a drop of insulin after 6 times, do not use the Insulin Aspart FlexPen and contact Novo Nordisk at 1-800-727-6500.
A small air bubble may remain at the needle tip, but it will not be injected.
Selecting your dose
Check and make sure that the dose selector is set at 0.
H.Turn the dose selector to the number of units you need to inject. The pointer should line up with your dose.
The dose can be corrected either up or down by turning the dose selector in either direction until the correct dose lines up with the pointer (see diagram H). When turning the dose selector, be careful not to press the push-button as insulin will come out.
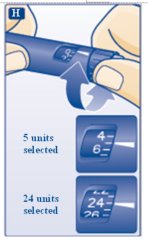
You cannot select a dose larger than the number of units left in the cartridge.
You will hear a click for every single unit dialed. Do not set the dose by counting the number of clicks you hear because you may get an incorrect dose.
- Δ Do not use the cartridge scale printed on the cartridge to measure your dose of insulin.
Giving the injection
Do the injection exactly as shown to you by your healthcare provider. Your healthcare provider should tell you if you need to pinch the skin before injecting. Wipe the skin with an alcohol swab and let the area dry.
Insulin Aspart can be injected under the skin (subcutaneously) of your stomach area, buttocks, upper legs (thighs), or upper arms.
Change (rotate) your injection sites within the area you choose for each dose to reduce your risk of getting lipodystrophy (pits in skin or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do notuse the same injection site for each injection. Do notinject where the skin has pits, is thickened, or has lumps. Do notinject where the skin is tender, bruised, scaly or hard, or into scars or damaged skin.
I.Insert the needle into your skin.
Inject the dose by pressing the push-button all the way in until the 0 lines up with the pointer (see diagram I). Be careful only to push the button when injecting.
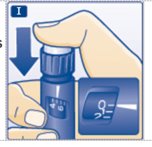
Turning the dose selector will not inject insulin.
J.Keep the needle in the skin for at least 6 seconds, and keep the push-button pressed all the way in until the needle has been pulled out from the skin (see diagram J). This will make sure that the full dose has been given.
You may see a drop of insulin at the needle tip. This is normal and has no effect on the dose you just received. If blood appears after you take the needle out of your skin, press the injection site lightly with an alcohol swab. Do not rub the area.
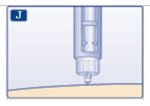
After the injection
Do not recap the needle.Recapping can lead to a needle stick injury. Remove the needle from the Insulin Aspart FlexPen after each injection and dispose of it. This helps to prevent infection, leakage of insulin, and will help to make sure you inject the right dose of insulin.
If you do not have a sharps container, carefully slip the needle into the outer needle cap. Safely remove the needle and throw it away as soon as you can.
- The used Insulin Aspart FlexPen may be thrown away in your household trash after you have removed the needle.
- Put your used needles in a FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at:
http://www.fda.gov/safesharpsdisposal.
Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container. - When there is not enough medicine left in your Insulin Aspart FlexPen for your prescribed dose, the Insulin Aspart FlexPen may be thrown away in your household trash after you have removed the needle. The Insulin Aspart FlexPen prevents the cartridge from being completely emptied. It is designed to deliver 300 units.
K. Put the pen cap on the Insulin Aspart FlexPen and store the Insulin Aspart FlexPen without the needle attached (see diagram K).
Storing without the needle attached helps prevent leaking, blocking of the needle, and air from entering the Pen.
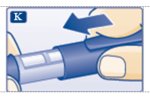
How should I store Insulin Aspart FlexPen?
- Do notfreeze Insulin Aspart. Do notuse Insulin Aspart if it has been frozen.
- Keep Insulin Aspart away from heat or light.
- Store the Insulin Aspart FlexPen without the needle attached.
- Until first use:
- Store unused Insulin Aspart FlexPen in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Unused FlexPen may be used until the expiration date printed on the label, if kept in the refrigerator.
- Unused Insulin Aspart FlexPen stored at room temperature should be thrown away after 28 days.
- In-use:
- Store the FlexPen you are currently using out of the refrigerator at room temperature below 86°F (30°C) for up to 28 days.
- The Insulin Aspart FlexPen you are using should be thrown away after 28 days, even if it still has insulin left in it.
Maintenance
For the safe and proper use of your FlexPen be sure to handle it with care. Avoid dropping your FlexPen as it may damage it. If you are concerned that your FlexPen is damaged, use a new one. You can clean the outside of your FlexPen by wiping it with a damp cloth. Do not soak or wash your FlexPen as it may damage it. Do not refill your FlexPen.
ΔRemove the needle from the Insulin Aspart FlexPen after each injection. This helps to ensure sterility, prevent leakage of insulin, and will help to make sure you inject the right dose of insulin for future injections.
ΔBe careful when handling used needles to avoid needle sticks and transfer of infectious diseases.
ΔKeep your Insulin Aspart FlexPen and needles out of the reach of children.
ΔUse Insulin Aspart FlexPen as directed to treat your diabetes.
Δ Do notshare your Insulin Aspart FlexPen or needles with other people. You may give other people a serious infection, or
get a serious infection from them.
ΔAlways use a new needle for each injection.
ΔNovo Nordisk is not responsible for harm due to using this insulin pen with products not recommended by Novo
Nordisk.
ΔAs a precautionary measure, always carry a spare insulin delivery device in case your Insulin Aspart FlexPen is lost or damaged.
ΔRemember to keep the disposable Insulin Aspart FlexPen with you. Do not leave it in a car or other location where it can get too hot or too cold.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Repackaged and Distributed By:
Remedy Repack, Inc.
625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
DRUG: Insulin Aspart
GENERIC: insulin aspart
DOSAGE: INJECTION, SOLUTION
ADMINSTRATION: INTRAVENOUS
NDC: 70518-2823-0
PACKAGING: 10 mL in 1 VIAL, GLASS
OUTER PACKAGING: 1 in 1 CARTON
ACTIVE INGREDIENT(S):
- INSULIN ASPART 100[iU] in 1mL
INACTIVE INGREDIENT(S):
- SODIUM PHOSPHATE, DIBASIC, DIHYDRATE
- GLYCERIN
- HYDROCHLORIC ACID
- METACRESOL
- PHENOL
- SODIUM CHLORIDE
- SODIUM HYDROXIDE
- ZINC