Uses
- •
- to “revive” someone during an overdose from many prescription pain medications or street drugs such as heroin
- •
- this medicine can save a life
Warning
- •
- When using this product some people may experience symptoms when they wake up, such as, shaking, sweating, nausea, or feeling angry. This is to be expected.
Directions
NARCAN®
Naloxone HCl Nasal Spray 4 mg
|
Step 1: CHECK if you suspect an overdose:
|
|
|
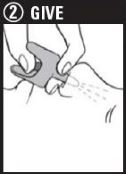
Step 2: Give 1st dose in the nose
|
|
|
Step 3: CALL 911
|
|
|
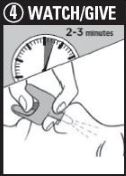
Step 4: WATCH & GIVE
|
|
|
Step 5: STAY
|
For opioid emergencies, call 911. For questions on NARCAN, call 1-844-4NARCAN (1-844-462-7226) or go to www.narcan.com.
©2023 Emergent Devices Inc. EMERGENT® and NARCAN® are registered trademarks of Emergent BioSolutions Inc, or its subsidiaries.
Other Information
- •
- store at room temperature or refrigerated, between 2°C to 25°C (36°F to 77°F)
- •
- do not freeze
- •
- avoid excessive heat above 40°C (104°F)
- •
- protect from light
- •
- the product is packaged in individually-sealed blisters.
Do not use if the blister is open or torn, or if the device appears damaged.
Inactive Ingredients
benzalkonium chloride, edetate disodium, hydrochloric acid, sodium chloride, water
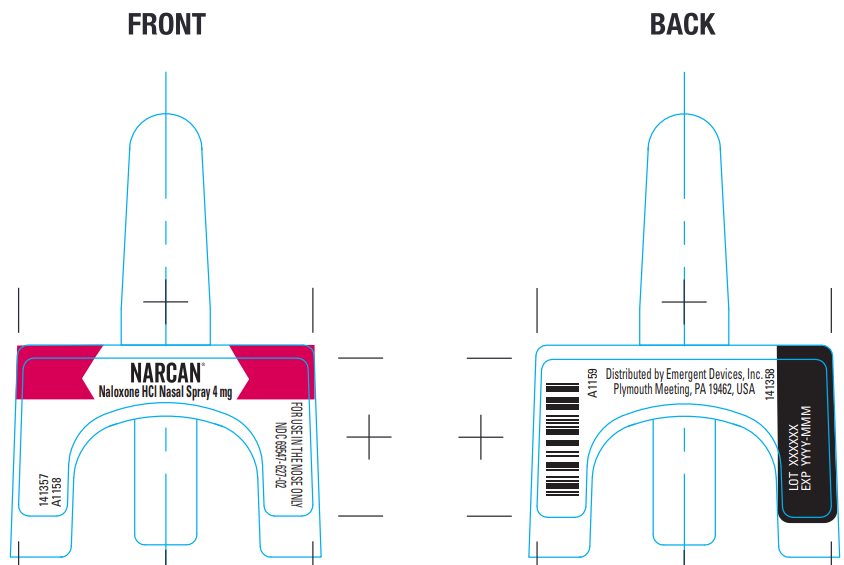
Package/Label Principal Display Panel – Carton Label
NARCAN®
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid Overdose
Original
Prescription
Strength
Easy to Use
Can Save a Life
Designed to
Rapidly Reverse
the Effects of a
Life-Threatening
Opioid Emergency
2 Single-Dose Nasal Spray Devices
0.003 fl oz (0.1 mL) each
For use in
nose only
Distributed by Emergent Devices Inc.
Plymouth Meeting, PA 19462 USA
©2023 Emergent Devices Inc. Plymouth Meeting, PA.
All rights reserved.
NARCAN® is a registered trademark of Emergent Operations
Ireland Limited.
NDC 69547-627-02
Package/Label Principal Display Panel - 4 mg Vial Package
NDC 69547-627-02
NARCAN®
Naloxone HCl Nasal Spray 4 mg
Emergency Treatment of Opioid Overdose
- •
- For use in the nose only
- •
- Do not test nasal spray device before use
- •
- 1 nasal spray device contains 1 dose of medicine
- •
- Each device sprays 1 time only
- •
- Store at room temperature or refrigerated,
between 2°C to 25°C (36°F to 77°F) - •
- Do not freeze
- •
- Avoid excessive heat above 40°C (104°F)
Lot XXXXXX EXP YYYY-MMM
Distributed by Emergent Devices Inc.
Plymouth Meeting, PA 19462 USA A1160