Label: MYCAPSSA- octreotide capsule, delayed release
- NDC Code(s): 10122-550-28
- Packager: Chiesi USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MYCAPSSA® safely and effectively. See full prescribing information for MYCAPSSA.
MYCAPSSA (octreotide) delayed-release capsules, for oral use
Initial U.S. Approval: 1988RECENT MAJOR CHANGES
Warnings and Precautions, Steatorrhea and Malabsorption of Dietary Fats (5.5) 07/2024
INDICATIONS AND USAGE
MYCAPSSA is a somatostatin analog indicated for long-term maintenance treatment in acromegaly patients who have responded to and tolerated treatment with octreotide or lanreotide (1).
DOSAGE AND ADMINISTRATION
- Take MYCAPSSA orally with a glass of water on an empty stomach, at least 1 hour before a meal or at least 2 hours after a meal (2.1).
- Initiate MYCAPSSA at a dosage of 40 mg daily, administered as 20 mg orally twice daily (2.2).
- Monitor insulin-like growth factor 1 (IGF-1) levels and patient's signs and symptoms every two weeks during the dose titration or as indicated (2.2).
- Titrate the MYCAPSSA dosage, based on IGF-1 levels and patient's signs and symptoms. Increase the dosage in increments of 20 mg (2.2).
- The maximum recommended dosage is 80 mg daily (2.2).
- Once the maintenance dosage of MYCAPSSA is achieved, monitor IGF-1 levels and patient's signs and symptoms monthly or as indicated (2.2).
- For patients with end-stage renal disease, initiate at a dosage of 20 mg orally once daily. Titrate and adjust the maintenance dosage based on IGF-1 levels, patient's signs and symptoms and tolerability (2.4).
DOSAGE FORMS AND STRENGTHS
Delayed-release capsules: 20 mg. (3)
CONTRAINDICATIONS
Hypersensitivity to octreotide or any of the components of MYCAPSSA. (4)
WARNINGS AND PRECAUTIONS
Cholelithiasis and Complications of Cholelithiasis: Monitor periodically. Discontinue if complications of cholelithiasis are suspected (5.1).
Hypoglycemia or Hyperglycemia: Monitor glucose and adjust antidiabetic treatment as needed (5.2).
Thyroid Function Abnormalities: Hypothyroidism may occur. Assess thyroid function periodically (5.3).
Cardiac Function: Bradycardia, arrhythmia, or conduction abnormalities may occur. Drugs that have bradycardia effects may need dosage adjustments (5.4, 7.2).
Steatorrhea and Malabsorption of Dietary Fats: New onset steatorrhea, stool discoloration, loose stools, abdominal bloating, and weight loss may occur. If new occurrence or worsening of these symptoms are reported, evaluate for potential pancreatic exocrine insufficiency (5.5)
Change in vitamin B12 levels: Monitor vitamin B12 levels during treatment (5.6).
ADVERSE REACTIONS
Most common adverse reactions (incidence >10 %) are nausea, diarrhea, headache, arthralgia, asthenia, hyperhidrosis, peripheral swelling, blood glucose increased, vomiting, abdominal discomfort, dyspepsia, sinusitis, osteoarthritis (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Chiesi Farmaceutici S.p.A. at 1-888-661-9260 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Proton Pump Inhibitors, H2-receptor Antagonists, or Antacids: may decrease bioavailability of MYCAPSSA and the MYCAPSSA dose may need to be increased (7).
- Cyclosporine: may have decreased bioavailability and require dose adjustment (7).
- Insulin and Antidiabetic Drugs: patients receiving insulin or antidiabetic drugs agents may require dose adjustment (7).
- Digoxin: exposure may be decreased and assessment of clinical response to digoxin should be performed (7).
- Lisinopril: bioavailability may be increased, monitor patient's blood pressure and adjust dose of lisinopril if needed (7).
- Levonorgestrel: counsel women to use an alternative non-hormonal method of contraception or a back-up method when MYCAPSSA is used with combined oral contraceptives (7).
- Bromocriptine: dose adjustment of bromocriptine may be necessary (7).
- Beta Blocker and Calcium Channel Blockers: dose adjustment of beta blockers or calcium channel blockers may be necessary (7).
- Drugs Metabolized by CYP 450 Enzymes: concomitant use with other drugs mainly metabolized by CYP3A4 that have a narrow therapeutic index (e.g., quinidine) should be used with caution and increased monitoring may be required (7).
USE IN SPECIFIC POPULATIONS
Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy (8.3).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Recommended Dosage, Titration, and Monitoring
2.3 Dosage Interruptions and Modifications
2.4 Recommended Dosage in Patients with End Stage Renal Disease
2.5 Dosage Modifications with Concomitant Use of Proton Pump Inhibitors, H2-receptor Antagonists, or Antacids
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
5.2 Hyperglycemia and Hypoglycemia
5.3 Thyroid Function Abnormalities
5.4 Cardiac Function Abnormalities
5.5 Steatorrhea and Malabsorption of Dietary Fats
5.6 Changes in Vitamin B12 Levels
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on MYCAPSSA
7.2 Effects of MYCAPSSA on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- Take MYCAPSSA orally with a glass of water on an empty stomach, at least 1 hour before a meal or at least 2 hours after a meal.
- Swallow MYCAPSSA capsules whole. Do not crush or chew the capsules.
2.2 Recommended Dosage, Titration, and Monitoring
- Initiate MYCAPSSA at a dosage of 40 mg daily, administered as 20 mg orally twice daily.
- Monitor insulin-like growth factor 1 (IGF-1) levels and patient's signs and symptoms every two weeks during the dose titration or as indicated.
- Titrate the MYCAPSSA dosage based on IGF-1 levels and patient's signs and symptoms. Increase the dosage in increments of 20 mg daily.
- For MYCAPSSA dosages of 60 mg daily, administer as 40 mg in the morning and 20 mg in the evening.
- For MYCAPSSA dosages of 80 mg daily, administer as 40 mg twice daily.
- The maximum recommended dosage of MYCAPSSA is 80 mg daily.
- Once the maintenance dosage of MYCAPSSA is achieved, monitor IGF-1 levels and patient's signs and symptoms monthly or as indicated.
2.3 Dosage Interruptions and Modifications
- If IGF-1 levels remain above the upper normal limit after treatment with the maximum recommended dosage of 80 mg daily or the patient cannot tolerate treatment with MYCAPSSA, consider discontinuing MYCAPSSA and switching patient to another somatostatin analog.
- Withdraw MYCAPSSA therapy periodically to assess disease activity. If IGF-1 levels increase and signs and symptoms recur, resume MYCAPSSA therapy.
2.4 Recommended Dosage in Patients with End Stage Renal Disease
For patients with end-stage renal disease, initiate MYCAPSSA at a dosage of 20 mg orally once daily. Titrate and adjust the maintenance dosage of MYCAPSSA based on IGF-1 levels, patient's signs and symptoms and tolerability [see Dosage and Administration (2.2, 2.3), Use in Specific Populations (8.6)].
2.5 Dosage Modifications with Concomitant Use of Proton Pump Inhibitors, H2-receptor Antagonists, or Antacids
Patients taking proton pump inhibitors, H2-receptor antagonists, or antacids concomitantly with MYCAPSSA may require increased dosages of MYCAPSSA [see Drug Interactions (7.1)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Hypersensitivity to octreotide or any of the components of MYCAPSSA. Anaphylactoid reactions, including anaphylactic shock, have been reported in patients receiving octreotide [see Adverse Reactions (6.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Cholelithiasis and Complications of Cholelithiasis
MYCAPSSA may inhibit gallbladder contractility and decrease bile secretion, which may lead to gallbladder abnormalities or sludge. Gallbladder-related adverse reactions have been reported in clinical trials in patients receiving MYCAPSSA. There have been postmarketing reports of cholelithiasis (gallstones) in patients taking somatostatin analogs resulting in complications, including cholecystitis, cholangitis, pancreatitis and requiring cholecystectomy [see Adverse Reactions (6)]. Monitor patients periodically. If complications of cholelithiasis are suspected, discontinue MYCAPSSA and treat appropriately.
5.2 Hyperglycemia and Hypoglycemia
MYCAPSSA alters the balance between the counter-regulatory hormones, insulin, glucagon, and growth hormone, which may result in hypoglycemia, or hyperglycemia, or diabetes mellitus. In clinical trials with MYCAPSSA, the following adverse reactions were reported: increased blood glucose (7%), hypoglycemia (4%), and diabetes mellitus (1%) [see Adverse Reactions (6.1)]. Blood glucose levels should be monitored when MYCAPSSA treatment is initiated, or when the dose is altered. Adjust antidiabetic treatment accordingly.
5.3 Thyroid Function Abnormalities
MYCAPSSA suppresses the secretion of thyroid-stimulating hormone, which may result in hypothyroidism. In clinical trials with MYCAPSSA, the following adverse reactions were reported: hypothyroidism (1%), increased TSH (1%), or decreased free T4 (1%) [see Adverse Reactions (6.1)]. Assess thyroid function periodically during treatment with MYCAPSSA.
5.4 Cardiac Function Abnormalities
Cardiac conduction abnormalities and other ECG changes including QT prolongation, axis shifts, early repolarization, low voltage, R/S transition, and early R wave progression, have occurred during treatment with octreotide. In MYCAPSSA clinical trials the following adverse reactions were reported: bradycardia (2%), conduction abnormalities (1%), and arrhythmias/tachycardia (2%) [see Adverse Reactions (6)]. These ECG changes may occur in patients with acromegaly. Dosage adjustments of concomitantly used drugs that have bradycardia effects (i.e. beta-blockers) may be necessary [see Drug Interactions (7.2)].
5.5 Steatorrhea and Malabsorption of Dietary Fats
New onset steatorrhea, stool discoloration and loose stools have been reported in patients receiving somatostatin analogs, including octreotide. Somatostatin analogs reversibly inhibit secretion of pancreatic enzymes and bile acids, which may result in malabsorption of dietary fats and subsequent symptoms of steatorrhea, loose stools, abdominal bloating, and weight loss. If new occurrence or worsening of these symptoms are reported in patients receiving MYCAPSSA, evaluate patients for potential pancreatic exocrine insufficiency and manage accordingly.
-
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Cholelithiasis and Complications of Cholelithiasis [see Warnings and Precautions (5.1)]
- Hyperglycemia and Hypoglycemia [see Warnings and Precautions (5.2)]
- Thyroid Function Abnormalities [see Warnings and Precautions (5.3)]
- Cardiac Function Abnormalities [see Warnings and Precautions (5.4)]
- Steatorrhea and Malabsorption of Dieatary Fats [see Warnings and Precautions (5.5)]
- Changes in Vitamin B12 Levels [see Warnings and Precautions (5.6)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
MYCAPSSA has been evaluated in patients with acromegaly in a placebo-controlled study [see Clinical Studies (14)] and an open-label baseline-controlled study. The data reflect exposure of 183 patients to MYCAPSSA for a mean duration of 29 weeks. In the overall study population, 56% were female and the average age of patients was 54.3 years. Adverse reactions occurring ≥ 5% and greater than placebo for the placebo-controlled study are presented in Table 1 and adverse reactions occurring ≥ 5% in the open-label study are presented in Table 2.
Table 1: Adverse Reactions Occurring ≥ 5% and Greater than Placebo in a Placebo-Controlled Study with MYCAPSSA in Acromegaly Patients MYCAPSSA %
(N=28)PLACEBO %
(N=28)- *
- Includes blood glucose increased, hyperglycemia and glycosylated hemoglobin increased
Diarrhea 29 21 Nausea 21 11 Blood glucose increased* 14 7 Vomiting 14 0 Abdominal discomfort 14 11 Dyspepsia 11 4 Sinusitis 11 0 Osteoarthritis 11 0 Urinary tract infection 7 4 Pain 7 0 Large intestine polyp 7 0 Cholelithiasis 7 4 Table 2: Adverse Reactions Occurring ≥ 5% in an Open-Label Study with MYCAPSSA in Acromegaly Patients MYCAPSSA %
(N=155)- *
- Includes blood glucose increased, hyperglycemia and impaired fasting glucose
Headache 33 Nausea 30 Arthralgia 26 Asthenia 22 Hyperhidrosis 21 Diarrhea 18 Peripheral swelling 16 Dyspepsia 8 Abdominal pain upper 8 Abdominal distension 7 Nasopharyngitis 7 Influenza 7 Blood glucose increased* 6 Vomiting 6 Flatulence 6 Back pain 6 Abdominal pain 5 Dizziness 5 Fatigue 5 Upper respiratory tract infection 5 Hypertension 5 Other Adverse Reactions
Gallbladder Abnormalities
In the placebo-controlled study, in patients treated with MYCAPSSA, acute cholecystitis occurred in 4% of patients.
In the open-label study, cholelithiasis occurred in 4.5% of patients and bile duct obstruction, bile duct stone, acute cholecystitis and jaundice occurred in 1% of patients each.
Hypoglycemia/Hyperglycemia
In the placebo-controlled study, 18% of patients treated with MYCAPSSA and 4% of patients treated with placebo developed at least one glucose value above the upper normal limit. All patients with abnormal glucose values were asymptomatic. Asymptomatic hypoglycemia was reported in 4% of patients.
In the open-label study 16% of patients developed a glucose value above the upper limit of normal. Asymptomatic hypoglycemia was reported in 4% and symptomatic hypoglycemia was reported in 1% of patients. Diabetes was reported in 1% of patients.
Hypothyroidism
In the open-label study, hypothyroidism, increased TSH, or decreased free T4 were reported in 1% of patients.
Cardiac
In the open-label study, bradycardia was reported in 2%, conduction abnormalities in 1%, and arrhythmias/tachycardia in 2% of patients.
Gastrointestinal
Gastrointestinal symptoms were the most commonly reported adverse reactions with MYCAPSSA.
In the placebo-controlled study, gastrointestinal adverse reactions were reported in 68% of patients treated with MYCAPSSA. These adverse reactions were diarrhea, nausea, vomiting, abdominal discomfort, dyspepsia, large intestinal polyp, abdominal pain, constipation, and flatulence. The adverse reactions were mild to moderate, occurred mostly during the initial 3 months of treatment, and resolved on treatment within a median duration of 8 days.
In the open-label study, gastrointestinal adverse reactions were reported in 57% of patients. Gastrointestinal adverse reactions occurring in ≥ 1% of patients were nausea, diarrhea, dyspepsia, abdominal pain, abdominal distention, vomiting, flatulence, constipation, gastroesophageal reflux disease, abdominal discomfort, frequent bowel movement, gastritis, hemorrhoids, dry mouth, and gastrointestinal motility disorder. Large intestinal polyp was reported in 1 patient. The adverse reactions were mostly mild to moderate, occurred during the initial 2 months of treatment, and resolved on treatment within a median of 13 days. Ten patients discontinued treatment due to gastrointestinal adverse reactions.
6.2 Immunogenicity
As with all therapeutic peptides, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other octreotide acetate products may be misleading.
No antibodies to the octreotide peptide from MYCAPSSA were detected in 149 patients assessed in the open label study throughout 13 months of treatment.
6.3 Postmarketing Experience
The following adverse reactions have been identified during the post-approval use of octreotide acetate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and lymphatic: pancytopenia, thrombocytopenia
- Cardiac: myocardial infarction, cardiac arrest, atrial fibrillation
- Ear and labyrinth: deafness
- Endocrine: diabetes insipidus, adrenal insufficiency in patients 18 months of age and under, pituitary apoplexy
- Eye: glaucoma, visual field defect, scotoma, retinal vein thrombosis
- Gastrointestinal: intestinal obstruction, peptic/gastric ulcer, abdomen enlarged
- General and administration site: generalized edema, facial edema
- Hepatobiliary: gallbladder polyp, fatty liver, hepatitis
- Immune: anaphylactoid reactions including anaphylactic shock
- Infections and infestations: appendicitis
- Laboratory abnormalities: increased liver enzymes, CK increased, creatinine increased
- Metabolism and nutrition: diabetes mellitus
- Musculoskeletal: arthritis, joint effusion, Raynaud's syndrome
- Nervous System: convulsions, aneurysm, intracranial hemorrhage, hemiparesis, paresis, suicide attempt, paranoia, migraines, Bell's palsy, aphasia
- Renal and urinary: renal failure, renal insufficiency
- Reproductive and breast: gynecomastia, galactorrhea, libido decrease, breast carcinoma
- Respiratory: status asthmaticus, pulmonary hypertension, pulmonary nodule, pneumothorax aggravated
- Skin and subcutaneous tissue: urticaria, cellulitis, petechiae
- Vascular: orthostatic hypotension, hematuria, gastrointestinal hemorrhage, arterial thrombosis of the arm
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on MYCAPSSA
Proton Pump Inhibitors, H2-receptor Antagonists, or Antacids Clinical Impact: Concomitant administration of MYCAPSSA with esomeprazole resulted in a decrease in the bioavailability for MYCAPSSA [See Clinical Pharmacology (12.3)]. Drugs that alter the pH of the upper GI tract (e.g., other proton pump inhibitors (PPIs), H2-receptor antagonists, and antacids) may alter the absorption of MYCAPSSA and lead to a reduction in bioavailability. Intervention: Co-administration of MYCAPSSA with PPIs, H2-blockers, or antacids may require increased doses of MYCAPSSA. 7.2 Effects of MYCAPSSA on Other Drugs
Cyclosporine Clinical Impact: Concomitant administration of MYCAPSSA with cyclosporine resulted in a decrease in cyclosporine bioavailability [see Clinical Pharmacology (12.3)]. Intervention: Adjustment of cyclosporine dose to maintain therapeutic levels may be necessitated. Insulin and Antidiabetic Drugs Clinical Impact: MYCAPSSA inhibits the secretion of insulin and glucagon. Intervention: Monitor blood glucose levels in diabetic patients upon MYCAPSSA initiation and subsequent dose adjustment. Patients receiving insulin or antidiabetic drugs agents may require dose adjustment of these therapeutic agents. Digoxin Clinical Impact: Concomitant administration of MYCAPSSA with digoxin resulted in a decrease in digoxin peak exposure [see Clinical Pharmacology (12.3)]. Intervention: Digoxin has a narrow therapeutic ratio and careful assessment of clinical response should be performed when digoxin is concomitantly administered with MYCAPSSA. Lisinopril Clinical Impact: Concomitant administration of MYCAPSSA increases lisinopril bioavailability [see Clinical Pharmacology (12.3)]. Intervention: Monitor patient's blood pressure and adjust the dosage of lisinopril if needed. Levonorgestrel Clinical Impact: Concomitant administration of MYCAPSSA with levonorgestrel decreases levonorgestrel bioavailability [see Clinical Pharmacology (12.3)]. Intervention: Decreased bioavailability may potentially diminish the effectiveness of combined oral contraceptives (COCs) or increase breakthrough bleeding. Counsel women to use an alternative non-hormonal method of contraception or a back-up method when MYCAPSSA is used with COCs. Bromocriptine Clinical Impact: Concomitant administration of MYCAPSSA with bromocriptine may increase the systemic exposure of bromocriptine [see Clinical Pharmacology (12.3)]. Intervention: Dose adjustment of bromocriptine may be necessary. Beta Blocker and Calcium Channel Blockers Clinical Impact: MYCAPSSA may cause bradycardia in acromegaly patients. Intervention: Patients receiving beta blockers or calcium channel blockers may require dose adjustments of these therapeutic agents. Drugs Metabolized by CYP 450 Enzymes Clinical Impact: Limited published data indicate that somatostatin analogs including MYCAPSSA may decrease the metabolic clearance of compounds known to be metabolized by cytochrome P450 enzymes, which may be due to the suppression of GH. Intervention: Concomitant use with other drugs mainly metabolized by CYP3A4 that have a narrow therapeutic index (e.g., quinidine) should be used with caution and increased monitoring may be required. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with octreotide acetate use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies have not been conducted with MYCAPSSA. No adverse developmental effects were observed with intravenous administration of octreotide to pregnant rats and rabbits during organogenesis at doses 7 and 13 times, respectively, the clinical dose based on octreotide injection body surface area. Transient growth retardation, with no impact on postnatal development, was observed in rat offspring from a pre- and post-natal study of octreotide at intravenous doses below the clinical dose based on octreotide injection body surface area (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received intravenous doses of octreotide up to 1 mg/kg/day during the period of organogenesis. A slight reduction in body weight gain was noted in pregnant rats at 0.1 and 1 mg/kg/day. There were no maternal effects in rabbits or embryo-fetal effects in either species up to the maximum dose tested. At 1 mg/kg/day in rats and rabbits, the dose multiple was approximately 7 and 13 times, respectively, the clinical dose based on octreotide injection body surface area.
In a pre- and post-natal development rat study at intravenous doses of 0.02-1 mg/kg/day, a transient growth retardation of the offspring was observed at all doses which was possibly a consequence of growth hormone inhibition by octreotide. The doses attributed to the delayed growth are below the clinical dose based on octreotide injection body surface area.
8.2 Lactation
Risk Summary
There is no information available on the presence of octreotide in human milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. Studies show that octreotide administered subcutaneously passes into the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for MYCAPSSA and any potential adverse effects on the breastfed child from MYCAPSSA or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as the therapeutic benefits of a reduction in GH levels and normalization of IGF-1 concentration in acromegalic females treated with octreotide may lead to improved fertility.
8.4 Pediatric Use
Safety and efficacy of MYCAPSSA in pediatric patients have not been established.
In post-marketing reports, serious adverse reactions, including hypoxia, necrotizing enterocolitis, and death, have been reported with octreotide injection use in pediatric patients, most notably in children under 2 years of age.
8.5 Geriatric Use
Clinical studies of octreotide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In MYCAPSSA clinical studies, 39 patients (21%) were age 65 years or over and 1 patient was age 75 years or over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
In patients with mild, moderate, or severe renal impairment there is no dose adjustment recommended for MYCAPSSA. There is a significant increase in octreotide exposure in patients with end stage renal disease (ESRD). Start patients with ESRD on MYCAPSSA 20 mg orally daily. Adjust the maintenance dose thereafter based on IGF-1 levels, patient's signs and symptoms, and tolerability [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Patients with liver cirrhosis and patients with fatty liver disease showed prolonged elimination of octreotide following subcutaneous administration of drug [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
A limited number of accidental overdoses of injectable octreotide acetate in adults has been reported. The doses ranged from 2.4 mg/day to 6 mg/day administered by continuous infusion or subcutaneously 1.5 mg three times a day. Adverse reactions in some patients included arrhythmia, hypotension, cardiac arrest, brain hypoxia, pancreatitis, hepatic steatosis, hepatomegaly, lactic acidosis, flushing, diarrhea, lethargy, weakness, and weight loss.
If overdose occurs, contact Poison Control (1-800-222-1222) for latest recommendations.
-
11 DESCRIPTION
MYCAPSSA delayed release capsules contain octreotide acetate, a somatostatin analog. Octreotide is known chemically as L-cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1-(hydroxy-methyl) propyl]-, cyclic (2→7)-disulfide; [R-(R*,R*)].
The molecular weight of octreotide is 1019.3 (free peptide, C49H66N10O10S2) and its amino acid sequence is:
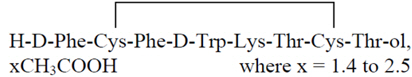
MYCAPSSA (octreotide) delayed-release capsules are enteric-coated capsules for oral use. Each capsule contains 20 mg of octreotide (provided as octreotide acetate). Octreotide is present as a salt with 1.4 to 2.5 molar equivalents of acetate. The capsules contain the following inactive ingredients: polyvinylpyrrolidone (PVP-12), sodium caprylate, magnesium chloride, polysorbate 80, glyceryl monocaprylate, glyceryl tricaprylate, gelatin, gelatin capsules, and Acryl-EZE® (methacrylate). The capsule is printed with "OT 20" in Opacode® black ink.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Octreotide exerts pharmacologic actions similar to the natural hormone somatostatin, but is a more potent inhibitor of GH, glucagon, and insulin than somatostatin. Like somatostatin, it also suppresses luteinizing hormone (LH) response to gonadotropin-releasing hormone (GnRH), decreases splanchnic blood flow, and inhibits release of serotonin, gastrin, vasoactive intestinal peptide, secretin, motilin, and pancreatic polypeptide.
12.2 Pharmacodynamics
In a single-dose PK study conducted in healthy volunteers, inhibition of GH (as measured by Cavg) was observed in all subjects receiving MYCAPSSA, as compared to their GH levels prior to MYCAPSSA.
In a study designed to assess the duration of MYCAPSSA-induced increased intestinal permeability, an increase in paracellular permeability was observed 2 hours after MYCAPSSA administration and returned to baseline by 5.5 hours after MYCAPSSA administration. MYCAPSSA-induced permeability is completely reversible within this timeframe.
MYCAPSSA maintained GH and IGF-1 levels in patients with acromegaly.
Single doses of octreotide acetate given subcutaneously have been shown to inhibit gallbladder contractility and to decrease bile secretion in normal volunteers. In clinical trials the incidence of gallstone or biliary sludge formation was markedly increased [see Warnings and Precautions (5.1)].
Octreotide acetate may cause clinically significant suppression of TSH [see Warnings and Precautions (5.3)].
12.3 Pharmacokinetics
Absorption
In healthy subjects, similar systemic exposure (AUC) was observed between a single dose oral administration of MYCAPSSA (20 mg octreotide acetate), and a single dose of subcutaneous Sandostatin IR (0.1 mg octreotide acetate). Peak octreotide levels (Cmax) were 33% lower following oral administration compared to the subcutaneous route. Absorption time was longer following oral administration compared to the subcutaneous route; peak concentrations were reached at a median of 1.67–2.5 hours after 20 mg MYCAPSSA administration compared to 0.5 hours for the subcutaneous administration.
In healthy subjects, after single-dose oral administration of MYCAPSSA, the systemic exposure of octreotide (Cmax, AUC0-24, and AUC0-inf) increased dose-proportionally at doses ranging from 3–40 mg.
In patients with acromegaly, there was a dose-related increase in the mean plasma octreotide concentrations after chronic administration of MYCAPSSA 40 mg (20 mg bid), 60 mg (40 mg AM / 20 mg PM), and 80 mg (40 mg AM / 40 mg PM) bid. Mean peak concentrations (Cmax) following chronic dosing were lower in patients with acromegaly (mean [CV%] = 2.51 ng/mL [80%] and 5.30 ng/mL [76%] at 20 and 40 mg bid, respectively) compared to single-dose peak concentrations observed in healthy subjects at the same dose (mean [CV%] = 3.62 [53%] and 8.21 ng/mL [88%] at 20 and 40 mg, respectively).
Distribution
In healthy volunteers, the distribution half-life (tα½) of octreotide acetate from plasma after subcutaneous administration was 0.2 h, the volume of distribution (Vdss) was estimated to be 13.6 L, and the total body clearance ranged from 7–10 L/hr. In blood, the distribution into the erythrocytes was found to be negligible and about 65% was bound in the plasma in a concentration-independent manner. Binding was mainly to lipoprotein and, to a lesser extent, to albumin.
In patients with acromegaly, the Vdss following subcutaneous administration was increased compared to healthy volunteers, estimated to be 21.6 L; mean peak concentrations were lower in acromegaly patients compared to healthy volunteers (2.8 ng/mL vs 5.2 ng/mL, respectively after 0.1 ng/mL dose).
Elimination
According to data obtained with the immediate-release octreotide subcutaneous injection, approximately 32% of the dose is excreted unchanged in the urine.
In healthy subjects, there was no effect of route of administration on octreotide elimination, and comparable mean elimination half-lives (t½) of 2.3 hours and 2.7 hours were demonstrated between subcutaneous injection and oral octreotide treatments, respectively.
In patients with acromegaly, elimination after chronic dosing was slightly slower than that seen in healthy volunteers, with mean apparent half-life values at steady state ranging from 3.2–4.5 hours across doses (20 mg, 40 mg, 60 mg, and 80 mg). Elimination is complete approximately 48 hours after the last dose in patients who have achieved steady-state plasma levels. Minimal accumulation (approximately 10%) was observed in patients after repeat administration of MYCAPSSA.
Specific Populations
Geriatric Patients
In patients 65 years of age and older, after subcutaneous administration of octreotide acetate, the half-life of octreotide increased significantly (46%) and clearance of octreotide decreased significantly (26%).
Patients with Renal Impairment
Exposure in patients with severe renal impairment was not substantially different from that of the matched controls. Following oral administration of a single dose of 20 mg MYCAPSSA to patients with severe renal impairment (eGFR 15–29 mL/min/1.73m2) and patients with end-stage renal disease (ESRD) requiring dialysis, patients with ESRD on dialysis had a 46% decrease in clearance with a corresponding 87% increase in AUC and 85% increase in t½ compared to matched healthy subjects. ESRD patients had higher mean plasma concentrations than did those with severe renal impairment with higher mean values for Cmax (9.30 ng/mL compared to 6.13 ng/mL in the matched controls), AUC0–t (68.0 h∙ng/mL compared to 32.2 h∙ng/mL in the matched controls), AUCinf (69.5 h∙ng/mL compared to 32.4 h∙ng/mL in the matched controls), and t½ (7.09 hr compared to 3.84 hr in the matched controls), consistent with the known effect of renal impairment on octreotide exposure [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
In patients with liver cirrhosis, after subcutaneous administration of octreotide acetate, prolonged elimination of drug was observed, with octreotide acetate t½ increasing from 1.9–3.7 hr and total body clearance decreasing from 7–10 L/hr to 5.9 L/hr, whereas patients with fatty liver disease showed t½ increased to 3.4 hr and total body clearance of 8.2 L/hr.
Drug Interactions
Limited published data indicate that somatostatin analogs including MYCAPSSA may decrease the metabolic clearance of compounds known to be metabolized by cytochrome P450 enzymes, which may be due to the suppression of GH [see Drug Interactions (7.2)].
Octreotide has been associated with alterations in nutrient absorption, so it may have an effect on absorption of orally administered drugs.
Table 3 Effect of Co-administered Drugs on MYCAPSSA Systemic Exposure Co-administered drug and dosing regimen MYCAPSSA Dose (mg) Mean Ratio (ratio with/without co-administered drug) No Effect=1.0 Change in AUC Change in Cmax Esomeprazole 40 mg QD on days 2-7 20 mg on Day 1 and 20 mg on Day 7 0.591
(0.40 – 0.88)20.551
(0.40 – 0.75)2Metoclopramide 20 mg 40 mg 0.91
(0.61 – 1.35)0.95
(0.62 – 1.44)Loperamide 4 mg 40 mg 0.97
(0.65 – 1.44)20.91
(0.59 – 1.39)21Clinically significant [see Dosage and Administration (2) and Drug Interactions (7.1, 7.2)]
2Mean ratio with 90% CI (with/without co-administered drug, e.g., 1= no change, 0.6 = 40% decrease,1.3=1.3-fold increase in exposure)Table 4 Effect of MYCAPSSA on Systemic Exposure of Co-administered Drugs Co-administered drug and dosing regimen MYCAPSSA Dose (mg)1 Mean Ratio (ratio with/without co-administered drug) No Effect=1.0 Change in AUC Change in Cmax Cyclosporine 300 mg 20 mg 0.382
(0.31 – 0.46)30.292
(0.22 – 0.37)3Digoxin 0.5 mg 40 mg 1.0
(0.94 – 1.13)30.632
(0.55 – 0.72)3Lisinopril 20 mg 40 mg 1.402
(1.21 – 1.61)31.502
(1.32 – 1.71)3Ethinyl Estradiol 0.06 mg 40 mg 0.94
(0.86 – 1.03)30.92
(0.83 – 1.01)3Levonorgestrel 0.3 mg 40 mg 0.762
(0.67 – 0.86)30.622
(0.54 – 0.71)31Single dose
2Clinically significant [see Dosage and Administration (2) and Drug Interactions (7.1, 7.2)]
3Mean ratio with 90% CI (with/without co-administered drug, e.g., 1= no change, 0.6 = 40% decrease, 1.5=1.5-fold increase in exposure) -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in laboratory animals have demonstrated no mutagenic potential of octreotide acetate.
No carcinogenicity studies have been conducted with MYCAPSSA. No carcinogenic potential was demonstrated in mice treated subcutaneously with octreotide acetate for 85–99 weeks at doses up to 2000 mcg/kg/day (8 times the clinical dose based on octreotide injection body surface area). In a 116-week subcutaneous study in rats administered octreotide acetate, a 27% and 12% incidence of injection-site sarcomas or squamous cell carcinomas was observed in males and females, respectively, at the highest dose level of 1250 mcg/kg/day (10 times the clinical dose based on octreotide injection body surface area) compared to an incidence of 8% to 10% in the vehicle-control groups. The increased incidence of injection-site tumors was most probably caused by irritation and the high sensitivity of the rat to repeated subcutaneous injections at the same site. There was also a 15% incidence of uterine adenocarcinomas in the 1250 mcg/kg/day females compared to 7% in the saline-control females and 0% in the vehicle-control females. The presence of endometritis coupled with the absence of corpora lutea, the reduction in mammary fibroadenomas, and the presence of uterine dilatation suggest that the uterine tumors were associated with estrogen dominance in the aged female rats, which does not occur in humans.
No fertility studies in animals have been conducted with MYCAPSSA. Injectable octreotide acetate did not impair fertility in rats at doses up to 1000 mcg/kg/day, which represents 7 times the clinical dose based on octreotide injection body surface area.
-
14 CLINICAL STUDIES
The efficacy of MYCAPSSA was established in a 9 month, randomized, double-blind, placebo-controlled study (NCT03252353) that enrolled 56 patients with acromegaly.
In the overall study population, 54% were female and the average age of patients was 55 years. 91% of patients were Caucasian, 5% Asian, 2% Black, and 2% Other. The percentage of patients with previous pituitary surgery was 88%. The baseline IGF-1 levels (the average of 2 assessments measured within 2 weeks of randomization) was 0.80 times ULN (range: 0.5–1.1 times ULN) in the patients treated with MYCAPSSA and 0.84 times ULN (range: 0.3–1.1 times ULN) in patients treated with the placebo.
In this study, patients initiated MYCAPSSA treatment twice daily 1 month after their last injection of somatostatin analogs. The starting dose was 40 mg (20 mg in the morning and 20 mg in the evening). Dose increase was allowed during dose titration to 60 mg (40 mg in the morning and 20 mg in the evening) and to a maximal dose of 80 mg daily (40 mg in the morning and 40 mg in the evening) until patients were deemed adequately controlled based on biochemical results and/or clinical judgement. Patients then maintained their target dose until end of treatment.
The primary efficacy endpoint was somatostatin dose-adjusted proportion of patients who maintain their biochemical response, defined as an IGF-1 levels less than or equal to the ULN at the end of 9 months of treatment. 58% of patients treated with MYCAPSSA vs. 19% of patients treated with placebo maintained their biochemical response.
25% of patients treated with MYCAPSSA required discontinuation of MYCAPSSA and treatment with other somatostatin analogs at some point during the 9-month study. Criteria for somatostatin analog rescue were IGF-1 levels ≥ 1.3 times ULN and exacerbation of acromegaly signs and symptoms on two consecutive assessments while treated for at least 2 weeks with 80 mg/day or other reasons such as adverse reactions or patient's decision.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Cholelithiasis and Complications of Cholelithiasis
Advise patients to contact their healthcare provider if they experience signs or symptoms of gallstones (cholelithiasis) or complications of cholelithiasis (e.g., cholecystitis, cholangitis and pancreatitis) [see Warnings and Precautions (5.1)].
Hypoglycemia and Hyperglycemia
Advise patients to contact their healthcare provider if they have problems with blood sugar levels, either hyperglycemia or hypoglycemia [see Warnings and Precautions (5.2)].
Thyroid Function Abnormalities
Inform patients that their thyroid function will be assessed periodically during treatment [see Warnings and Precautions (5.3)].
Cardiac Function Abnormalities
Inform patients to contact the health care provider in case they notice irregular heartbeat [see Warnings and Precautions (5.4)].
Steatorrhea and Malabsorption of Dietary Fats
Advise patients to contact their healthcare provider if they experience new or worsening symptoms of steatorrhea, stool discoloration, loose stools, abdominal bloating, weight loss [see Warnings and Precautions (5.5)].
Changes in Vitamin B12 Levels
Inform patients that Vitamin B12 levels may be monitored during the treatment [see Warnings and Precautions (5.5)].
Females and Males of Reproductive Potential
Inform female patients that treatment with MYCAPSSA may result in unintended pregnancy [see Use in Specific Populations (8.3)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
Patient Information
MYCAPSSA® [my (as in sky)-cap-sah]
(octreotide)
delayed-release capsules, for oral useThis Patient Information has been approved by the U.S. Food and Drug Administration. Approved: 07/2024 What is MYCAPSSA? - MYCAPSSA is an oral prescription medicine used in the long-term maintenance treatment of acromegaly in people for whom initial treatment with octreotide or lanreotide has been effective and tolerated.
- It is not known if MYCAPSSA is safe and effective in children.
Do not take MYCAPSSA if you: - are allergic to octreotide acetate or any of the ingredients in MYCAPSSA. MYCAPSSA can cause a serious allergic reaction including anaphylactic shock. Stop taking MYCAPSSA right away and get emergency help if you have any of these symptoms:
- swelling of your tongue, throat, lips, eyes or face
- severe itching of the skin with rash or raised bumps
- chest pain
- trouble swallowing or breathing
- feeling faint
- rapid heart beat
See the end of this leaflet for a complete list of ingredients in MYCAPSSA. Before you take MYCAPSSA, tell your healthcare provider about all of your medical conditions, including if you: - have liver cirrhosis or liver problems
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if MYCAPSSA will harm your unborn baby. MYCAPSSA may increase your chance of becoming pregnant.
- are breastfeeding or plan to breastfeed. It is not known if MYCAPSSA passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take MYCAPSSA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. MYCAPSSA may affect the way other medicines work, and other medicines may affect how MYCAPSSA works. Especially tell your healthcare provider if you take oral contraceptives. Use an alternative non-hormonal method of contraception or a back-up method while taking MYCAPSSA. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine. How should I take MYCAPSSA? - Read the detailed "Instructions for Use" at the end of this Patient Information about the right way to take MYCAPSSA.
- Take MYCAPSSA exactly as your healthcare provider tells you to take it.
- Take MYCAPSSA with a glass of water on an empty stomach.
- Take MYCAPSSA at least 1 hour before a meal or at least 2 hours after a meal (for example, you could take your morning dose 1 hour before breakfast and your evening dose at bedtime).
Swallow the capsules whole. Do not crush or chew the capsules before swallowing. What are the possible side effects of MYCAPSSA? - gallbladder problems. MYCAPSSA may cause problems with the gallbladder. Tell your healthcare provider if you have sudden pain in your upper right stomach (abdomen), sudden pain in your right shoulder or between your shoulder blades, yellowing of your skin or the whites of your eyes, fever with chills, nausea
- blood sugar problems. MYCAPSSA may cause you to have high blood sugar (hyperglycemia), low blood sugar (hypoglycemia), or diabetes. Tell your healthcare provider if you have problems with high or low blood sugar. Your healthcare provider will check your blood sugar when you start taking MYCAPSSA or when your dose is changed.
- thyroid problems. MYCAPSSA may keep your thyroid from releasing thyroid hormones leading to hypothyroidism. Your thyroid function will be checked regularly during your treatment with MYCAPSSA.
- heart rhythm problems. Tell your healthcare provider if you have an irregular heartbeat (your heart is not beating normally).
- fatty stool. MYCAPSSA may cause your body to have issues with absorbing dietary fats. Tell your healthcare provider if you have any new or worsening symptoms including fatty stools, changes in the color of your stools, loose stools, stomach (abdominal) bloating or weight loss.
- low vitamin B12 levels in your blood. Your healthcare provider may check your vitamin B12 levels during treatment with MYCAPSSA.
The most common side effects of MYCAPSSA include: - headache
- nausea
- diarrhea
- joint pain
- weakness
- sweating a lot
These are not all the possible side effects of MYCAPSSA. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store MYCAPSSA? - Before first use, store unopened wallets of MYCAPSSA in a refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze.
- After first use, store opened wallets at room temperature between 68°F to 77°F (20°C to 25°C) for up to 1 month.
Keep MYCAPSSA and all medicines out of the reach of children. General information about the safe and effective use of MYCAPSSA. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information. Do not use MYCAPSSA for a condition for which it was not prescribed. Do not give MYCAPSSA to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about MYCAPSSA that is written for health professionals. What are the ingredients in MYCAPSSA? Active ingredient: octreotide acetate Inactive ingredients: polyvinylpyrrolidone (PVP-12), sodium caprylate, magnesium chloride, polysorbate 80, glyceryl monocaprylate, glyceryl tricaprylate, gelatin, gelatin capsules, and Acryl-EZE® (methacrylate). Chiesi Farmaceutici S.p.A.,
Parma, ItalyFor more information about MYCAPSSA call the medical information department at 1-888-661-9260 or us.medical@chiesi.com or go to www.MYCAPSSA.com and select patient information. -
INSTRUCTIONS FOR USE
MYCAPSSA® [my (as in sky)-cap-sah]
(octreotide)
delayed-release capsules, for oral use
Read this Instructions for Use before you start taking MYCAPSSA and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. Talk to your healthcare provider or pharmacist if you have any questions about how to use MYCAPSSA.
Important information:
- Each MYCAPSSA wallet contains twenty-eight 20-mg capsules. The number of wallets required in a 28-day period depends on your prescribed dose.
How to Use the MYCAPSSA Wallet
- Each MYCAPSSA wallet has a locking mechanism that helps to keep the medicine away from children.
- Become familiar with using the MYCAPSSA wallet so you will know how to use it the right way.
To open the wallet:
Step 1. With your left thumb, gently press the tip of the release button on the left side of the wallet (see Figure A).
Step 2. While holding the release button, grasp the medicine card at the notch on the right side and pull it out (see Figure A).
Step 3. Unfold the medicine card (see Figure A).
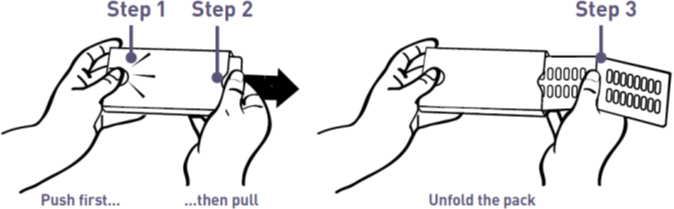
Figure A: How to Open the MYCAPSSA Wallet
How to Remove a Capsule from the MYCAPSSA Wallet
Capsules need to be removed carefully, because if they are cracked or broken they may not be as effective. Follow these instructions to easily remove capsules without damaging them.
- Place the tip of a thumb at the edge of a capsule's plastic cavity (see Figure B).
- Gently push the capsule until it is removed. Collect the removed capsule in your hand.
- Do not use two thumbs to push a capsule as this could damage it.
- Do not press the middle of a capsule. This could also damage it.
- If a capsule is cracked or broken, throw it away (discard it) and remove another capsule.
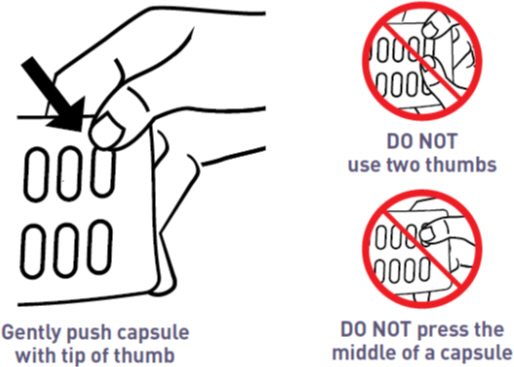
Figure B: How to Remove a Capsule from the Medicine Card Inside the MYCAPSSA Wallet
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Approved: 01/2024 -
PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack
Always take with a glass of water on an empty stomach at least
1 hour before a meal or at least 2 hours after a meal.Mycapssa®
(octreotide) delayed-release capsules20 mg*
2
28 Capsules
*Each capsule contains octreotide 20 mg (provided as octreotide acetate).
Acetate composition varies. See prescribing information.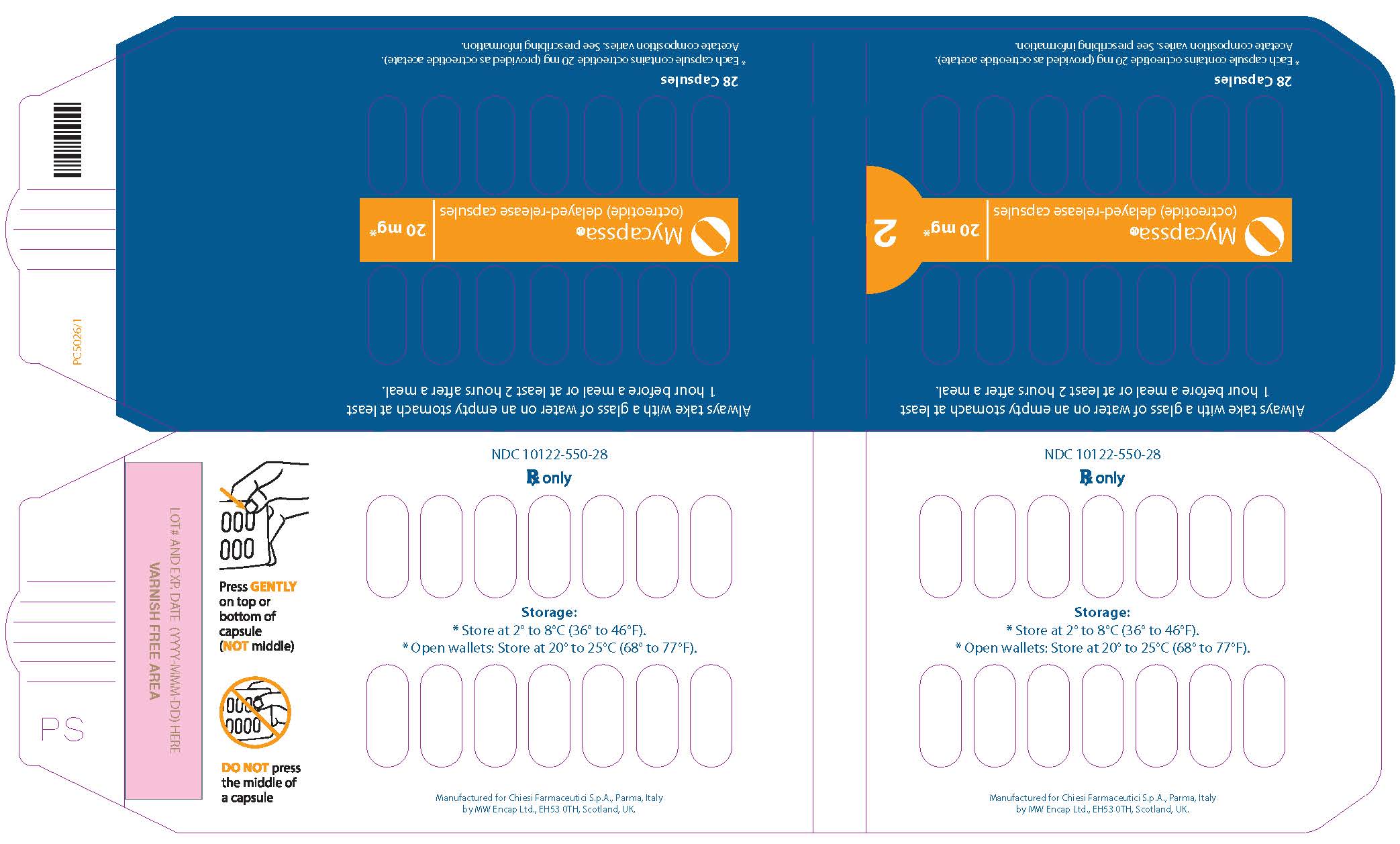
-
PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack Dose Pack
NDC 10122-550-28
Mycapssa®
(octreotide) delayed-release capsules20 mg*
Rx only
*Each capsule contains octreotide 20 mg (provided
as octreotide acetate). Acetate composition varies.
See prescribing information.
Keep out of reach of children.1
PRESS
& HOLD
HEREPULL OUT HERE
❶
PRESS❷
PULLOPENING AND CLOSING INSTRUCTIONS
- Use left thumb to push the button gently
- While holding the button down, pull out the medication card
- Press out to take capsule(s)
- Slide medication card back to lock
28 capsules
Always take with a glass of water on an empty stomach at least
1 hour before a meal or at least 2 hours after a meal.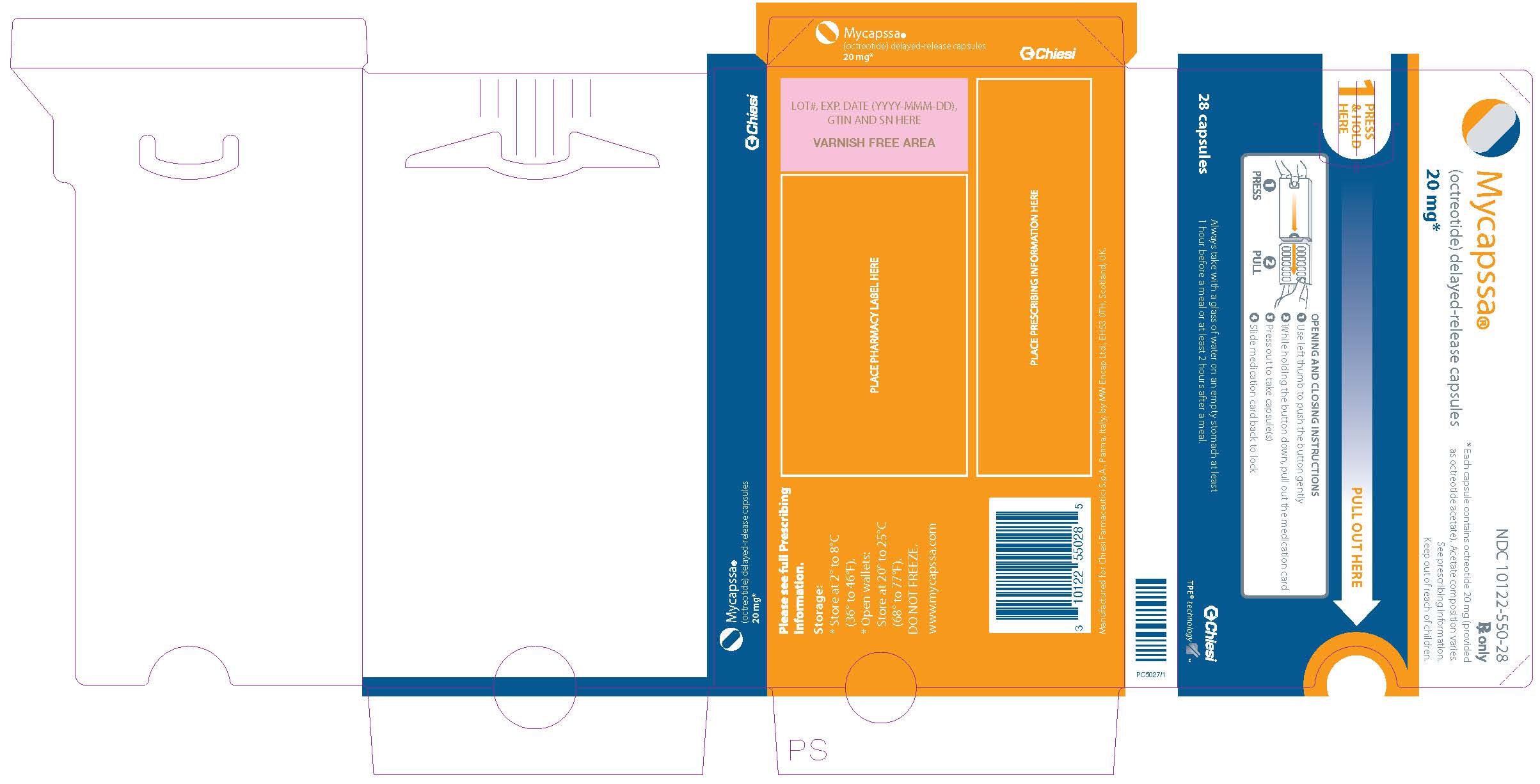
-
INGREDIENTS AND APPEARANCE
MYCAPSSA
octreotide capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:10122-550 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octreotide (UNII: RWM8CCW8GP) (Octreotide - UNII:RWM8CCW8GP) Octreotide 20 mg Inactive Ingredients Ingredient Name Strength POVIDONE K12 (UNII: 333AG72FWJ) sodium caprylate (UNII: 9XTM81VK2B) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) polysorbate 80 (UNII: 6OZP39ZG8H) glyceryl monocaprylate (UNII: TM2TZD4G4A) TRICAPRILIN (UNII: 6P92858988) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMER (UNII: NX76LV5T8J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BICARBONATE (UNII: 8MDF5V39QO) TALC (UNII: 7SEV7J4R1U) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color white (Imprinted black) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code OT20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10122-550-28 4 in 1 DOSE PACK 07/06/2020 1 7 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208232 07/06/2020 Labeler - Chiesi USA, Inc. (088084228) Establishment Name Address ID/FEI Business Operations Almac Pharma Services Limited 233170864 label(10122-550) , pack(10122-550) Establishment Name Address ID/FEI Business Operations Assia Chemical Industries Ltd. - Teva Tech Site 649323474 api manufacture(10122-550) Establishment Name Address ID/FEI Business Operations Lyophilization Services of New England, Inc. (LSNE) 028180765 manufacture(10122-550) Establishment Name Address ID/FEI Business Operations MW ENCAP LIMITED 578075491 analysis(10122-550) , manufacture(10122-550) Establishment Name Address ID/FEI Business Operations Plantex Ltd. 600023907 api manufacture(10122-550)


