Label: FENOGLIDE- fenofibrate tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 54868-6118-0 - Packager: Physicians Total Care, Inc.
- This is a repackaged label.
- Source NDC Code(s): 59630-495
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 12, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FENOGLIDE safely and effectively. See full prescribing information for FENOGLIDE. FENOGLIDE (fenofibrate) tablet for oral use Initial U.S. Approval: 1993
INDICATIONS AND USAGE
Fenoglide is a peroxisome proliferator receptor alpha (PPARα) activator indicated:
- as an adjunct to diet to reduce elevated LDL-C, Total-C, TG, and Apo B, and to increase HDL-C in patients with primary hyperlipidemia or mixed dyslipidemia when response to diet and non-pharmacological interventions alone has been inadequate. (1.1)
- as an adjunct to diet to treat patients with hypertriglyceridemia. Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually obviate the need for pharmacologic intervention. (1.2).
Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in a large, randomized controlled trial of patients with type 2 diabetes mellitus. (1.3).
DOSAGE AND ADMINISTRATION
- Primary hyperlipidemia and mixed dyslipidemia: 120 mg /day (2.1)
- Hypertriglyceridemia: 40 to 120 mg/day; the dose should be adjusted according to patient response (2.2)
- Renally impaired patients: 40 mg/day; the dose should be increased according to the effect on renal function and lipid levels (2.4)
To increase absorption of Fenoglide, take with food.
DOSAGE FORMS AND STRENGTHS
- 40 mg: White to off-white oval tablets. Debossed "FLO".
- 120 mg: White to off-white oval tablets. Debossed "FHI".
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Fenofibrate can increase serum transaminases. Liver tests should be periodically monitored (5.1).
- Fenofibrate increases cholesterol excretion into the bile, leading to risk of cholelithiasis. If cholelithiasis is suspected, gallbladder studies are indicated. (5.2)
- Exercise caution in concomitant treatment with coumarin anticoagulants. Reduce the dosage of coumarin to maintain the prothrombin time/INR at the desired level to prevent bleeding complications (5.3).
- Myopathy and rhabdomyolysis have been reported in patients taking fenofibrate. The risk for serious muscle toxicity appears to be increased in elderly patients and in patients with diabetes, renal failure, or hypothyroidism (5.4)
- Fenofibrate reversibly increases serum creatinine levels (5.5)
ADVERSE REACTIONS
The most common adverse reactions (> 2% and at least 1% greater than placebo) are abnormal liver tests, increased AST, increased ALT, increased CPK, and rhinitis (6.1).To report SUSPECTED ADVERSE REACTIONS, contact Sciele Drug Safety Department at 1-800-849-9707 ext. 1454 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
Revised: 5/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Hyperlipidemia and Mixed Dyslipidemia
1.2 Hypertriglyceridemia
1.3 General Guidelines
2 DOSAGE AND ADMINISTRATION
2.1 Hyperlipidemia and Mixed Dyslipidemia
2.2 Hypertriglyceridemia
2.3 Renally Impaired Patients
2.4 Elderly Patients
2.5 General Dosing Information
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Liver Tests
5.2 Cholelithiasis
5.3 Concomitant Coumarin Anticoagulants
5.4 Skeletal Muscle
5.5 Serum Creatinine
5.6 Mortality and Coronary Heart Disease Morbidity
5.7 Pancreatitis
5.8 Venothromboembolic Disease
5.9 Hypersensitivity Reactions
5.10 Hematologic Changes
5.11 Concomitant HMG-CoA Reductase Inhibitors
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Coumarin Anticoagulants
7.2 Cyclosporine
7.3 HMG-CoA Reductase Inhibitors
7.4 Bile-Acid Resins
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXIC
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Hyperlipidemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
14.2 Hypertriglyceridemia
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Enter section text here
1.1 Hyperlipidemia and Mixed Dyslipidemia
Fenoglide is indicated as adjunctive therapy to diet to reduce elevated LDL-C, Total-C, Triglycerides, and Apo B, and to increase HDL-C in adult patients with primary hyperlipidemia or mixed dyslipidemia. Lipid-altering agents should be used in addition to a diet restricted in saturated fat and cholesterol when response to diet and non-pharmacological interventions alone has been inadequate.
1.2 Hypertriglyceridemia
Fenoglide is also indicated as adjunctive therapy to diet for treatment of adult patients with hypertriglyceridemia. Improving glycemic control in diabetic patients showing fasting chylomicronemia will usually reduce fasting triglycerides and eliminate chylomicronemia thereby obviating the need for pharmacologic intervention.
1.3 General Guidelines
Fenofibrate was not shown to reduce coronary heart disease morbidity and mortality in a large, randomized controlled trial of patients with type 2 diabetes mellitus. [see Warnings and Precautions(5.6).]
Markedly elevated levels of serum triglycerides (e.g. >2,000 mg/dL) may increase the risk of developing pancreatitis. The effect of Fenoglide therapy on reducing this risk has not been adequately studied.
Drug therapy is not indicated for patients who have elevations of chylomicrons and plasma triglycerides, but who have normal levels of very low density lipoprotein (VLDL). Inspection of plasma refrigerated for 14 hours is helpful in distinguishing these patients from those with elevated VLDL.2
-
2 DOSAGE AND ADMINISTRATION
Enter section text here
2.2 Hypertriglyceridemia
The initial dose is 40 to 120 mg per day. Dosage should be individualized according to patient response, and should be adjusted if necessary following repeat lipid determinations at 4 to 8 week intervals. The maximum dose is 120 mg per day.
2.3 Renally Impaired Patients
Treatment with Fenoglide should be initiated at a dose of 40 mg/day in patients with mild to moderately impaired renal function, and increased only after evaluation of the effects on renal function and lipid levels at this dose. The use of Fenoglide should be avoided in patients with severely impaired renal function.
2.4 Elderly Patients
Dose selection for the elderly should be made on the basis of renal function. [See Use in Specific Populations (8.5).]
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- Fenoglide is contraindicated in patients with severe renal dysfunction, including those receiving dialysis [see Clinical Pharmacology (12.3)]
- Fenoglide is contraindicated in patients with active liver disease, including those with primary biliary cirrhosis and unexplained persistent liver function abnormalities [see Warnings and Precautions(5.1)]
- Fenoglide is contraindicated in patients with gallbladder disease [see Warnings and Precautions (5.2)]
- Fenoglide is contraindicated in nursing mothers [see Use in Specific Populations (8.3)]
- Fenoglide is contraindicated in patients who have a known hypersensitivity to fenofibrate, such as severe skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis. [see Warnings and Precautions (5.9)]
-
5 WARNINGS AND PRECAUTIONS
Enter section text here
5.1 Liver Tests
Fenofibrate at doses equivalent to 87 mg to 130 mg fenofibrate per day [at the highest dose, comparable to Fenoglide, 120 mg] can increase serum transaminases [AST (SGOT) or ALT (SGPT)].
In a pooled analysis of 10 placebo-controlled trials, increases to >3 times the upper limit of normal occurred in 5.3% of patients taking fenofibrate versus 1.1% of patients treated with placebo.
When transaminase determinations were followed either after discontinuation of treatment or during continued treatment, a return to normal limits was usually observed. The incidence of increases in transaminases related to fenofibrate therapy appear to be dose related. In an 8-week dose-ranging study, the incidence of ALT or AST elevations to at least three times the upper limit of normal was 13% in patients receiving dosages equivalent to 87 mg to 130 mg fenofibrate per day and was 0% in those receiving dosages equivalent to 43 mg or less fenofibrate per day, or placebo. Hepatocellular, chronic active and cholestatic hepatitis have been reported after exposures of weeks to several years. In extremely rare cases, cirrhosis has been reported in association with chronic active hepatitis.
Regular periodic monitoring of liver tests, including serum ALT (SGPT) should be performed for the duration of therapy with Fenoglide, and therapy discontinued if enzyme levels persist above three times the normal limit.
5.2 Cholelithiasis
Fenofibrate increases cholesterol excretion into the bile, leading to risk of cholelithiasis. If cholelithiasis is suspected, gallbladder studies are indicated. Fenoglide therapy should be discontinued if gallstones are found.
5.3 Concomitant Coumarin Anticoagulants
Caution should be exercised when anticoagulants are given in conjunction with Fenoglide because of the potentiation of coumarin-type anti-coagulants in prolonging the prothrombin time/INR. The dosage of the anticoagulant should be reduced to maintain the prothrombin time/INR at the desired level to prevent bleeding complications. Frequent prothrombin time/INR determinations are advisable until it has been definitely determined that the prothrombin time/INR has stabilized. [See Drug Interactions (7.1).]
5.4 Skeletal Muscle
Treatment with fenofibrate increases risk of myopathy. Treatment with fenofibrate increases risk of rhabdomyolysis, usually in patients with impaired renal function. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevations of creatine phosphokinase (CPK) levels.
Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and fenofibrate therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed.
5.5 Serum Creatinine
Elevations in serum creatinine have been reported in patients on fenofibrate. These elevations tend to return to baseline following discontinuation of fenofibrate. The clinical significance of these observations is unknown.
5.6 Mortality and Coronary Heart Disease Morbidity
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a 5-year randomized, placebo-controlled study of 9,795 patients with type 2 diabetes mellitus treated with fenofibrate. Fenofibrate demonstrated a non-significant 11% relative reduction in the primary outcome of coronary heart disease events (hazard ratio [HR] 0.89, 95% CI 0.75-1.05, p=0.16) and a significant 11% reduction in the secondary outcome of total cardiovascular disease events (HR 0.89 [0.80-0.99], p=0.04). There was a non-significant 11% (HR 1.11 [0.95, 1.29], p=0.18) and 19% (HR 1.19 [0.90, 1.57], p=0.22) increase in total and coronary heart disease mortality, respectively, with fenofibrate as compared to placebo.
In the Coronary Drug Project, a large study of post myocardial infarction of patients treated for 5 years with clofibrate, there was no difference in mortality seen between the clofibrate group and the placebo group.
In a study conducted by the World Health Organization (WHO), 5000 subjects without known coronary artery disease were treated with placebo or clofibrate for 5 years and followed for an additional one year. There was a statistically significant, higher age-adjusted all-cause mortality in the clofibrate group compared with the placebo group (5.70% vs. 3.96%, p= less than 0.01). Excess mortality was due to a 33% increase in non-cardiovascular causes, including malignancy, post-cholecystectomy complications, and pancreatitis. This appeared to confirm the higher risk of gallbladder disease seen in clofibrate-treated patients studied in the Coronary Drug Project.
The Helsinki Heart Study was a large (n=4,081) study of middle-aged men without a history of coronary artery disease. Subjects received either placebo or gemfibrozil for 5 years, with a 3.5 year open extension afterward. Total mortality was numerically higher in the gemfibrozil randomization group but did not achieve statistical significance (p=0.19, 95% confidence interval for relative risk G:P=0.91-1.64). Although cancer deaths trended higher in the gemfibrozil group (p=0.11), cancers (excluding basal cell carcinoma) were diagnosed with equal frequency in both study groups. Due to the limited size of the study, the relative risk of death from any cause was not shown to be different than that seen in the 9 year follow-up data from the WHO study (RR=1.29).
A secondary prevention component of the Helsinki Heart Study enrolled middle-aged men excluded from the primary prevention study because of known or suspected coronary heart disease. Subjects received gemfibrozil or placebo for 5 years. Although cardiac deaths trended higher in the gemfibrozil group, this was not statistically significant (hazard ratio 2.2, 95% confidence interval: 0.94-5.05).
5.7 Pancreatitis
Pancreatitis has been reported in patients taking fenofibrate. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
5.8 Venothromboembolic Disease
In the FIELD trial, pulmonary embolus (PE) and deep vein thrombosis (DVT) were observed at higher rates in the fenofibrate- than the placebo-treated group. Of 9,795 patients enrolled in FIELD, there were 4,900 in the placebo group and 4,895 in the fenofibrate group. For DVT, there were 48 events (1%) in the placebo group and 67 (1%) in the fenofibrate group (p = 0.074); and for PE, there were 32 (0.7%) events in the placebo group and 53 (1%) in the fenofibrate group (p = 0.022).
In the Coronary Drug Project, a higher proportion of the clofibrate group experienced definite or suspected fatal or nonfatal pulmonary embolism or thrombophlebitis than the placebo group (5.2% vs. 3.3% at five years; p less than 0.01).
5.9 Hypersensitivity Reactions
Acute hypersensitivity reactions including severe skin rashes requiring patient hospitalization and treatment with steroids have occurred rarely during treatment with fenofibrate, including rare spontaneous reports of Stevens-Johnson syndrome, and toxic epidermal necrolysis.
5.10 Hematologic Changes
Mild to moderate hemoglobin, hematocrit, and white blood cell decreases have been observed in patients following initiation of fenofibrate therapy.
5.11 Concomitant HMG-CoA Reductase Inhibitors
The combined use of fenofibric acid derivatives, particularly gemfibrozil, and HMG-CoA reductase inhibitors results in an increased risk of rhabdomyolysis and myoglobinuria leading in a high proportion of cases to acute renal failure.
The combined use of Fenoglide and HMG-CoA reductase inhibitors should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination. [see Drug Interactions (7.3).]
-
6 ADVERSE REACTIONS
To report SUSPECTED ADVERSE REACTIONS, contact Sciele Drug Safety Department at 1-800-849-9707 ext. 1454 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
6.1 Clinical Studies Experience
Table 1. Adverse Reactions Reported by 2% or More of Patients Treated with Fenofibrate and Greater than Placebo During the Double-Blind, Placebo-Controlled Trials BODY SYSTEM
Adverse Reaction
Fenofibrate*
(N=439)Placebo
(N=365)
BODY AS A WHOLE
Abdominal Pain
4.6%
4.4%
Back Pain
3.4 %
2.5%
Headache
3.2%
2.7%
DIGESTIVE
Nausea
2.3%
1.9%
Constipation
2.1%
1.4%
INVESTIGATIONS
Abnormal Liver Tests
7.5%
1.4%
Increased AST
3.4%
0.5%
Increased ALT
3.0%
1.6%
Increased Creatine Phosphokinase
3.0%
1.4%
RESPIRATORY
Respiratory Disorder
6.2%
5.5%
Rhinitis
2.3%
1.1%
* Dosage equivalent to 130 mg fenofibrate
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of fenofibrate: myalgia, rhabdomyolysis, increased creatinine phosphokinase, pancreatitis, increased alanine aminotransaminase, increased aspartate aminotransaminase, renal failure, muscle spasms, acute renal failure, hepatitis, cirrhosis, nausea, abdominal pain, anemia, headache, arthralgia, and asthenia. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a casual relationship to drug exposure.
-
7 DRUG INTERACTIONS
Enter section text here
7.1 Coumarin Anticoagulants
Caution should be exercised when coumarin anticoagulants are given in conjunction with Fenoglide. The dosage of the anticoagulants should be reduced to maintain the prothrombin time/INR at the desired level to prevent bleeding complications. Frequent prothrombin time/INR determinations are advisable until it has been definitely determined that the prothrombin time/INR has stabilized. [see Concomitant Coumarin Anticoagulants (5.3).]
7.2 Cyclosporine
Because cyclosporine can produce nephrotoxicity with decreases in creatinine clearance and rises in serum creatinine, and because renal excretion is the primary elimination route of fibrate drugs including Fenoglide, there is a risk that an interaction will lead to deterioration of renal function. The benefits and risks of using Fenoglide with immunosuppressants and other potentially nephrotoxic agents should be carefully considered, and the lowest effective dose employed.
7.3 HMG-CoA Reductase Inhibitors
The combined use of fenofibric acid derivatives, particularly gemfibrozil, and HMG-CoA reductase inhibitors results in an increased risk of rhabdomyolysis and myoglobinuria leading in a high proportion of cases to acute renal failure. [See Concomitant HMG-CoA Reductase Inhibitors (5.11)]
The combined use of Fenoglide and HMG-CoA reductase inhibitors should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination.
In a single-dose drug interaction study in 23 healthy adults the concomitant administration of fenofibrate and pravastatin resulted in no clinically important difference in the pharmacokinetics of fenofibric acid, pravastatin, or its active metabolite 3α-hydroxy iso-pravastatin when compared to either drug given alone.
-
8 USE IN SPECIFIC POPULATIONS
Enter section text here
8.1 Pregnancy
Fenofibrate is classified as pregnancy category C. Fenofibrate has been shown to be embryocidal and teratogenic in rats when given in doses 7 to 10 times the maximum recommended human dose and embryocidal in rabbits when given at 9 times the maximum recommended human dose (on the basis of mg/meter2 surface area). There are no adequate and well-controlled studies in pregnant women. Fenofibrate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. [see Nonclinical Toxicology [13]).
8.3 Nursing Mothers
Fenofibrate should not be used in nursing mothers. Because of the potential for tumorigenicity seen in animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug.
8.5 Geriatric Use
Fenofibric acid exposure is not influenced by age. However, elderly patients have a higher incidence of renal impairment. Dose selection for the elderly should be made on the basis of renal function.
8.6 Renal Impairment
Fenofibric acid is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Fenofibrate should be avoided in patients with severe renal impairment and dose reduction is required in patients with mild to moderate renal impairment.
-
10 OVERDOSAGE
There is no specific treatment for overdose with Fenoglide. General supportive care of the patient is indicated, including monitoring of vital signs and observation of clinical status, should an overdose occur. If indicated, elimination of unabsorbed drug should be achieved by emesis or gastric lavage; usual precautions should be observed to maintain the airway. Because fenofibrate is highly bound to plasma proteins, hemodialysis should not be considered.
-
11 DESCRIPTION
Fenoglide (fenofibrate) Tablets, is a lipid regulating agent available as tablets for oral administration. Each tablet contains 40 mg or 120 mg fenofibrate. The chemical name for fenofibrate is 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoic acid, 1-methylethyl ester with the following structural formula:
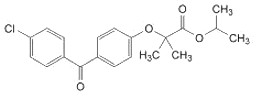
The empirical formula is C20H21O4Cl and the molecular weight is 360.83; fenofibrate is insoluble in water. The melting point is 79° to 82°C. Fenofibrate is a white solid which is stable under ordinary conditions.
Inactive Ingredients: Each tablet contains lactose monohydrate, NF; Polyethylene Glycol 6000, NF; Poloxamer 188, NF; and magnesium stearate, NF.
-
12 CLINICAL PHARMACOLOGY
Enter section text here
12.1 Mechanism of Action
The effects of fenofibric acid seen in clinical practice have been explained in vivo in transgenic mice and in vitro in human hepatocyte cultures by the activation of peroxisome proliferator activated receptor α (PPARα).
Through this mechanism, fenofibrate increases lipolysis and elimination of triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of apoprotein C-III (an inhibitor of lipoprotein lipase activity). The resulting fall in triglycerides produces an alteration in the size and composition of LDL from small, dense particles (which are thought to be atherogenic due to their susceptibility to oxidation), to large buoyant particles. These larger particles have a greater affinity for cholesterol receptors and are catabolized rapidly. Activation of PPARα also induces an increase in the synthesis of apoproteins A-I,A-II and HDL-cholesterol.
Fenofibrate also reduces serum uric acid levels in hyperuricemic and normal individuals by increasing the urinary excretion of uric acid.
12.2 Pharmacodynamics
A variety of clinical studies have demonstrated that elevated levels of total cholesterol (total-C), low density lipoprotein cholesterol (LDL-C), and apolipoprotein B (apo B), an LDL membrane complex, are risk factors for human atherosclerosis. Similarly, decreased levels of high density lipoprotein cholesterol (HDL-C) and its transport complex, apolipoprotein A (apo AI and apo AII) are risk factors for the development of atherosclerosis. Epidemiologic investigations have established that cardiovascular morbidity and mortality vary directly with the level of total-C, LDL-C, and triglycerides, and inversely with the level of HDL-C. The independent effect of raising HDL-C or lowering triglycerides (TG) on the risk of cardiovascular morbidity and mortality has not been determined.
Fenofibric acid, the active metabolite of fenofibrate, produces reductions in total cholesterol, LDL cholesterol, apolipoprotein B, total triglycerides, and triglyceride-rich lipoprotein (VLDL) in treated patients. In addition, treatment with fenofibrate results in increases in high density lipoprotein (HDL) and apoproteins apo AI and apo AII.
12.3 Pharmacokinetics
Plasma concentrations of fenofibric acid after single-dose administration of Fenoglide (fenofibrate) Tablets, 120 mg are equivalent to those of Fenofibrate 130 mg capsules under high-fat conditions.
A high-fat meal did not affect the fenofibric acid AUC after Fenoglide administration but did increase the mean Cmax by 44% compared to fasting conditions.
-
Absorption: The absolute bioavailability of
fenofibrate cannot be determined as the compound is virtually insoluble in
aqueous media suitable for injection. However, Fenoglide is well absorbed from
the gastrointestinal tract. Following oral administration in healthy volunteers,
approximately 60% of a single dose of radiolabelled fenofibrate appeared in
urine, primarily as fenofibric acid and its glucuronate conjugate, and 25% was
excreted in the feces. Peak plasma levels of fenofibric acid from Fenoglide
occur, on average, within 2 to 3 hours after administration.
Doses of three Fenoglide (fenofibrate) Tablets, 40 mg are considered to be equivalent to single doses of Fenoglide (fenofibrate) Tablets, 120 mg. - Distribution: In healthy volunteers, steady-state plasma levels of fenofibric acid were shown to be achieved within a week of dosing and did not demonstrate accumulation across time following multiple dose administration. Serum protein binding was approximately 99% in normal and hyperlipidemic subjects.
-
Metabolism: Following oral administration,
fenofibrate is rapidly hydrolyzed by esterases to the active metabolite,
fenofibric acid; no unchanged fenofibrate is detected in plasma.
Fenofibric acid is primarily conjugated with glucuronic acid and then excreted in urine. A small amount of fenofibric acid is reduced at the carbonyl moiety to a benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine.
In vivo metabolism data indicate that neither fenofibrate nor fenofibric acid undergo oxidative metabolism (e.g., cytochrome P450) to a significant extent. -
Excretion: After absorption, fenofibrate is
mainly excreted in the urine in the form of metabolites, primarily fenofibric
acid and fenofibric acid glucuronide. After administration of radiolabelled
fenofibrate, approximately 60% of the dose appeared in the urine and 25% was
excreted in the feces.
Fenofibrate acid from Fenoglide is eliminated with a half-life of 23 hours, allowing once daily administration in a clinical setting. - Geriatrics: In elderly volunteers 77 to 87 years of age, the oral clearance of fenofibric acid following a single oral dose of fenofibrate was 1.2 L/h, which compares to 1.1 L/h in young adults. This indicates that a similar dosage regimen can be used in the elderly, without increasing accumulation of the drug or metabolites. [See Dosage and Administration (2.4) and Use in Specific Populations (8.5).]
- Pediatrics: Fenoglide has not been investigated in adequate and well-controlled trials in pediatric patients.
- Gender: No pharmacokinetic difference between males and females has been observed for fenofibrate.
- Race: The influence of race on the pharmacokinetics of fenofibrate has not been studied; however, fenofibrate is not metabolized by enzymes known for exhibiting inter-ethnic variability. Therefore, inter-ethnic pharmacokinetic differences are very unlikely.
- Renal Insufficiency: The pharmacokinetics of fenofibric acid was examined in patients with mild, moderate, and severe renal impairment. Patients with severe renal impairment (creatinine clearance [CrCl] less than or equal to 30 mL/min or estimated glomerular filtration rate [eGFR] less than 30 mL/min/1.73m2) showed 2.7-fold increase in exposure for fenofibric acid and increased accumulation of fenofibric acid during chronic dosing compared to that of healthy subjects. Patients with mild to moderate renal impairment (CrCl 30-80 mL/min or eGFR 30-59 mL/min/1.73m2) had similar exposure but an increase in the half-life for fenofibric acid compared to that of healthy subjects. Based on these findings, the use of Fenoglide should be avoided in patients who have severe renal impairment and dose reduction is required in patients having mild to moderate renal impairment. [See Dosage and Administration (2.3).]
- Hepatic Insufficiency: No pharmacokinetic studies have been conducted in patients having hepatic insufficiency.
-
Drug-Drug Interactions: In vitro studies using human liver microsomes indicate that
fenofibrate and fenofibric acid are not inhibitors of cytochrome (CYP) P450
isoforms CYP3A4, CYP2D6, CYP2E1, or CYP1A2. They are weak inhibitors of CYP2C19
and CYP2A6, and mild-to-moderate inhibitors of CYP2C9 at therapeutic
concentrations.
Potentiation of coumarin-type anticoagulants has been observed with prolongation of the prothrombin time/INR. [See Concomitant Coumarin Anticoagulants (5.3).]
Bile-acid resins have been shown to bind other drugs given concurrently. Therefore, fenofibrate should be taken at least 1 hour before or 4 to 6 hours after a bile acid binding resin to avoid impeding its absorption. [See Drug Interactions (7.4).]
-
Absorption: The absolute bioavailability of
fenofibrate cannot be determined as the compound is virtually insoluble in
aqueous media suitable for injection. However, Fenoglide is well absorbed from
the gastrointestinal tract. Following oral administration in healthy volunteers,
approximately 60% of a single dose of radiolabelled fenofibrate appeared in
urine, primarily as fenofibric acid and its glucuronate conjugate, and 25% was
excreted in the feces. Peak plasma levels of fenofibric acid from Fenoglide
occur, on average, within 2 to 3 hours after administration.
-
13 NONCLINICAL TOXIC
Enter section text here
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month study in rats (10, 45, and 200 mg/kg; 0.3, 1, and 6 times the maximum recommended human dose on the basis of mg/meter2 of surface area), the incidence of liver carcinoma was significantly increased at 6 times the maximum recommended human dose in males and females. A statistically significant increase in pancreatic carcinomas occurred in males at 1 and 6 times the maximum recommended human dose; there were also increases in pancreatic adenomas and benign testicular interstitial cell tumors at 6 times the maximum recommended human dose in males. In a second 24-month study in a different strain of rats (doses of 10 and 60 mg/kg; 0.3 and 2 times the maximum recommended human dose based on mg/meter2 surface area), there were significant increases in the incidence of pancreatic acinar adenomas in both sexes and increases in interstitial cell tumors of the testes at 2 times the maximum recommended human dose.
A comparative carcinogenicity study was done in rats comparing three drugs: fenofibrate (10 and 70 mg/kg; 0.3 and 1.6 times the maximum recommended human dose), clofibrate (400 mg/kg; 1.6 times the human dose), and gemfibrozil (250 mg/kg; 1.7 times the human dose) (multiples based on mg/meter2 surface area). Pancreatic acinar adenomas were increased in males and females on fenofibrate; hepatocellular carcinoma and pancreatic acinar adenomas were increased in males and hepatic neoplastic nodules in females treated with clofibrate; hepatic neoplastic nodules were increased in males and females treated with gemfibrozil while testicular interstitial cell tumors were increased in males on all three drugs.
In a 21-month study in mice at doses of 10, 45, and 200 mg/kg (approximately 0.2, 0.7 and 3 times the maximum recommended human dose on the basis of mg/meter2 surface area), there were statistically significant increases in liver carcinoma at 3 times the maximum recommended human dose in both males and females. In a second 18-month study at the same doses, there was a significant increase in liver carcinoma in male mice and liver adenoma in female mice at 3 times the maximum recommended human dose.
Electron microscopy studies have demonstrated peroxisomal proliferation following fenofibrate administration to the rat. An adequate study to test for peroxisome proliferation in humans has not been done, but changes in peroxisome morphology and numbers have been observed in humans after treatment with other members of the fibrate class when liver biopsies were compared before and after treatment in the same individual.
Fenofibrate has been demonstrated to be devoid of mutagenic potential in the following tests: Ames, mouse lymphoma, chromosomal aberration, and unscheduled DNA synthesis.
Administration of 9 times the maximum recommended human dose of fenofibrate to female rats before and throughout gestation caused 100% of dams to delay delivery and resulted in a 60% increase in post-implantation loss, a decrease in litter size, a decrease in birth weight, a 40% survival of pups at birth, a 4% survival of pups as neonates, and a 0% survival of pups to weaning, and an increase in spina bifida.
Administration of 10 times the maximum recommended human dose to female rats on days 6-15 of gestation caused an increase in gross, visceral and skeletal findings in fetuses (domed head/hunched shoulders/rounded body/abnormal chest, kyphosis, stunted fetuses, elongated sternal ribs, malformed sternebrae, extra foramen in palatine, misshapen vertebrae, supernumerary ribs).
Administration of 7 times the maximum recommended human dose to female rats from day 15 of gestation through weaning caused a delay in delivery, a 40% decrease in live births, a 75% decrease in neonatal survival, and decreases in pup weight, at birth as well as on days 4 and 21 post-partum.
Administration of 9 and 18 times the maximum recommended human dose to female rabbits caused abortions in 10% of dams at 9 times and 25% of dams at 18 times the maximum recommended human dose and death of 7% of fetuses at 18 times the maximum recommended human dose.
-
14 CLINICAL STUDIES
Enter section text here
14.1 Hyperlipidemia (Heterozygous Familial and Nonfamilial) and Mixed Dyslipidemia
The effects of fenofibrate at a dose equivalent to 120 mg Fenoglide per day were assessed from four randomized, placebo-controlled, double-blind, parallel-group studies including patients with the following mean baseline lipid values: total-C 306.9 mg/dL; LDL-C 213.8 mg/dL; HDL-C 52.3 mg/dL; and triglycerides 191.0 mg/dL. Fenofibrate therapy lowered LDL-C, Total-C, and the LDL-C/HDL-C ratio. Fenofibrate therapy also lowered triglycerides and raised HDL-C (see Table 2).
* Duration of study treatment was 3 to 6 months.Table 2. Mean Percent Change in Lipid Parameters at End of Treatment* Treatment Group
Total-C
LDL-C
HDL-C
TG
Pooled Cohort
Mean baseline lipid values (n=646)
306.9 mg/dL
213.8 mg/dL
52.3 mg/dL
191.0 mg/dL
All FEN (n=361)
-18.7%† -20.6%† +11.0%† -28.9%† Placebo (n=285)
-0.4%
-2.2%
+0.7%
+7.7%
Baseline LDL-C greater than 160 mg/dL
and TG less than 150 mg/dL (Type IIa)
Mean baseline lipid values (n=334)
307.7 mg/dL
227.7 mg/dL
58.1 mg/dL
101.7 mg/dL
All FEN (n=193)
-22.4%† -31.4%† +9.8%† -23.5%† Placebo (n=141)
+0.2%
-2.2%
+2.6%
+11.7%
Baseline LDL-C greater than 160 mg/dL
and TG less than or equal to 150 mg/dL (Type IIb)
Mean baseline lipid values (n=242)
312.8 mg/dL
219.8 mg/dL
46.7 mg/dL
231.9 mg/dL
All FEN (n=126)
-16.8%† -20.1%† +14.6%† -35.9%† Placebo (n=116)
-3.0%
-6.6%
+2.3%
+0.9%
†
p= less than 0.05 vs. placebo
In a subset of the subjects, measurements of apo B were conducted. Fenofibrate treatment significantly reduced apo B from baseline to endpoint as compared with placebo (-25.1% vs. 2.4%, p less than 0.0001, n=213 and 143 respectively).
14.2 Hypertriglyceridemia
The effects of fenofibrate on serum triglycerides were studied in two randomized, double-blind, placebo-controlled clinical trials of 147 hypertriglyceridemic patients. Patients were treated for eight weeks under protocols that differed only in that one entered patients with baseline triglyceride (TG) levels of 500 to 1500 mg/dL, and the other TG levels of 350 to 500 mg/dL. In patients with hypertriglyceridemia and normal cholesterolemia with or without hyperchylomicronemia, treatment with fenofibrate at dosages equivalent to 120 mg Fenoglide (fenofibrate) Tablets per day decreased primarily very low density lipoprotein (VLDL) triglycerides and VLDL cholesterol. Treatment of patients with elevated triglycerides often results in an increase of low density lipoprotein (LDL) cholesterol (see Table 3).
*Table 3. Effects of Fenofibrate in Patients With Hypertriglyceridemia Study 1
Pla
cebo
Feno
fibrate
Baseline TG levels 350 to 499 mg/dL
N
Baseline
(Mean)
Endpoint
(Mean)
% Change
(Mean)
N
Baseline
(Mean)
Endpoint
(Mean)
% Change
(Mean)
Triglycerides
28
449
450
-0.5
27
432
223
-46.2* VLDL Triglycerides
19
367
350
2.7
19
350
178
-44.1* Total Cholesterol
28
255
261
2.8
27
252
227
-9.1* HDL Cholesterol
28
35
36
4
27
34
40
19.6* LDL Cholesterol
28
120
129
12
27
128
137
14.5 VLDL Cholesterol
27
99
99
5.8
27
92
46
-44.7* Study 2
Pla
cebo
Feno
fibrate
Baseline TG levels 500 to 1500 mg/dL
N
Baseline
(Mean)
Endpoint
(Mean)
% Change
(Mean)
N
Baseline
(Mean)
Endpoint
(Mean)
% Change
(Mean)
Triglycerides 44
710
750
7.2
48
726
308
-54.5* VLDL Triglycerides 29
537
571
18.7
33
543
205
-50.6* Total Cholesterol 44
272
271
0.4
48
261
223
-13.8* HDL Cholesterol 44
27
28
5.0
48
30
36
22.9* LDL Cholesterol 42
100
90
-4.2
45
103
131
45.0* VLDL Cholesterol 42
137
142
11.0
45
126
54
-49.4*
=p less than 0.05 vs. placebo
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FENOGLIDE
fenofibrate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:54868-6118(NDC:59630-495) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FENOFIBRATE (UNII: U202363UOS) (FENOFIBRATE - UNII:U202363UOS) FENOFIBRATE 120 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) POLYETHYLENE GLYCOL 6000 (UNII: 30IQX730WE) POLOXAMER 188 (UNII: LQA7B6G8JG) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to off-white) Score no score Shape OVAL Size 18mm Flavor Imprint Code FHI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54868-6118-0 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022118 04/28/2010 Labeler - Physicians Total Care, Inc. (194123980) Establishment Name Address ID/FEI Business Operations Physicians Total Care, Inc. 194123980 repack, relabel



