Label: EMVERM- mebendazole tablet, chewable
- NDC Code(s): 64896-669-30
- Packager: Amneal Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated August 23, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use EMVERM® safely and effectively. See full prescribing information for EMVERM®.
EMVERM® (mebendazole) chewable tablets, for oral use
Initial U.S. Approval: 1974INDICATIONS AND USAGE
EMVERM® is an anthelmintic indicated for the treatment of patients two years of age and older with gastrointestinal infections caused by:
-
Ancylostoma duodenale (hookworm),
-
Ascaris lumbricoides (roundworm),
-
Enterobius vermicularis (pinworm),
-
Necator americanus (hookworm), and
-
Trichuris trichiura (whipworm), (1).
DOSAGE AND ADMINISTRATION
Adults and Pediatrics: The tablet may be chewed, swallowed, or crushed and mixed with food (2).
Pinworm (enterobiasis)
Whipworm (trichuriasis)
Roundworm (ascariasis)
Hookworm
Dose
1 tablet once
1 tablet morning and evening for 3 consecutive days
1 tablet morning and evening for 3 consecutive days
1 tablet morning and evening for 3 consecutive days
DOSAGE FORMS AND STRENGTHS
-
Chewable Tablet: 100 mg (3)
CONTRAINDICATIONS
- Patients with a known hypersensitivity to the drug or its excipients (4)
WARNINGS AND PRECAUTIONS
- Risk of Convulsions: Convulsions in infants below the age of 1 year have been reported (5.1).
- Hematologic Effects: Neutropenia and agranulocytosis have been reported in patients receiving mebendazole at higher doses and for prolonged duration. Monitor blood counts in these patients (5.2).
- Metronidazole and Serious Skin Reactions: Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) have been reported with the concomitant use of mebendazole and metronidazole. Avoid concomitant use of mebendazole and metronidazole (5.3).
ADVERSE REACTIONS
Adverse reactions reported in clinical trials were anorexia, abdominal pain, diarrhea, flatulence, nausea, vomiting and rash (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Pharmaceuticals at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2021
-
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Convulsions
5.2 Hematologic Effects
5.3 Metronidazole Drug Interaction and Serious Skin Reactions
6 ADVERSE REACTIONS
6.1 Clinical Studies
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dosage for EMVERM® is described in Table 1 below. The same dosage schedule applies to adults and pediatric patients two years of age and older. The tablet may be chewed, swallowed, or crushed and mixed with food.
Table 1: Dosage of EMVERM in Adult and Pediatric Patients (two years of age and older)
Pinworm (enterobiasis)
Whipworm (trichuriasis)
Roundworm (ascariasis)
Hookworm
Dose
1 tablet once
1 tablet morning and evening for 3 consecutive days
1 tablet morning and evening for 3 consecutive days
1 tablet morning and evening for 3 consecutive days
If the patient is not cured three weeks after treatment, a second course of treatment is advised. No special procedures, such as fasting or purging, are required.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Convulsions
Although EMVERM® is approved for use in children two years of age and older, convulsions have been reported in infants below the age of 1 year during post-marketing experience with mebendazole, including EMVERM® [see Adverse Reactions (6.2) and Use in Specific Populations (8.4)].
5.2 Hematologic Effects
Agranulocytosis and neutropenia have been reported with mebendazole use at higher doses and for more prolonged durations than is recommended for the treatment of soil-transmitted helminth infections. Monitor blood counts if EMVERM® is used at higher doses or for prolonged duration.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of mebendazole was evaluated in 6276 subjects who participated in 39 clinical trials for treatment of single or mixed parasitic infections of the gastrointestinal tract. In these trials, the formulations, dosages and duration of mebendazole treatment varied. Adverse reactions reported in mebendazole-treated subjects from the 39 clinical trials are shown in Table 2 below.
Table 2: Adverse Reactions Reported in Mebendazole-treated Subjects from 39 Clinical Trials*
Adverse Reaction(s)
Gastrointestinal Disorders
Anorexia
Abdominal Pain
Diarrhea
Flatulence
Nausea
Vomiting
Skin and Subcutaneous Tissue Disorders
Rash
*Includes mebendazole formulations, dosages and treatment duration other than EMVERM® 100 mg tablet
6.2 Postmarketing Experience
The following adverse reactions have been identified in adult and pediatric patients postmarketing with mebendazole formulations and dosages other than the EMVERM® 100 mg chewable tablet. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Table 3: Adverse Reactions Identified During Postmarketing Experience with Mebendazole*
Adverse Reaction(s) Blood and Lymphatic System Disorders Agranulocytosis, Neutropenia Immune System Disorders Hypersensitivity including anaphylactic reactions Nervous System Disorders Convulsions, Dizziness Hepatobiliary Disorders Hepatitis, Abnormal liver tests Renal and Urinary Disorders Glomerulonephritis Skin and Subcutaneous Tissue Disorders Toxic epidermal necrolysis, Stevens-Johnson syndrome, Exanthema, Angioedema, Urticaria, Alopecia
*Includes mebendazole formulations, dosages and treatment duration other than EMVERM® 100 mg chewable tablets
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available published literature on mebendazole use in pregnant women has not reported a clear association between mebendazole and a potential risk of major birth defects or miscarriages [see Data]. There are risks to the mother and fetus associated with untreated helminthic infection during pregnancy [see Clinical Considerations].
In animal reproduction studies, adverse developmental effects (i.e., skeletal malformations, soft tissue malformations, decreased pup weight, embryolethality) were observed when mebendazole was administered to pregnant rats during the period of organogenesis at single oral doses as low as 10 mg/kg (approximately 0.5-fold the total daily maximum recommended human dose [MRHD]). Maternal toxicity was present at the highest of these doses [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risks
Untreated soil transmitted helminth infections in pregnancy are associated with adverse outcomes including maternal iron deficiency anemia, low birth weight, neonatal and maternal death.
Data
Human Data
Several published studies, including prospective pregnancy registries, case-control, retrospective cohort, and randomized controlled studies, have reported no association between mebendazole use and a potential risk of major birth defects or miscarriage. Overall, these studies did not identify a specific pattern or frequency of major birth defects with mebendazole use. However, these studies cannot definitely establish the absence of any mebendazole-associated risk because of methodological limitations, including recall bias, confounding factors and, in some cases, small sample size or exclusion of first trimester mebendazole exposures.
Animal Data
Embryo-fetal developmental toxicity studies in rats revealed no adverse effects on dams or their progeny at doses up to 2.5 mg/kg/day on gestation days 6–15 (the period of organogenesis). Dosing at ≥10 mg/kg/day resulted in a lowered body weight gain and a decreased pregnancy rate. Maternal toxicity, including body weight loss in one animal and maternal death in 11 of 20 animals, was seen at 40 mg/kg/day. At 10 mg/kg/day, increased embryo-fetal resorption (100% were resorbed at 40 mg/kg/day), decreased pup weight and increased incidence of malformations (primarily skeletal) were observed. Mebendazole was also embryotoxic and teratogenic in pregnant rats at single oral doses during organogenesis as low as 10 mg/kg (approximately 0.5-fold the total daily MRHD, based on mg/m2).
In embryo-fetal developmental toxicity studies in mice dosed on gestation days 6–15, doses of 10 mg/kg/day and higher resulted in decreased body weight gain at 10 and 40 mg/kg/day and a higher mortality rate at 40 mg/kg/day. At doses of 10 mg/kg/day (approximately 0.2-fold the total daily MRHD, based on mg/m2) and higher, embryo-fetal resorption increased (100% at 40 mg/kg) and fetal malformations, including skeletal, cranial, and soft tissue anomalies, were present. Dosing of hamsters and rabbits did not result in embryotoxicity or teratogenicity at doses up to 40 mg/kg/day (1.6 to 3.9-fold the total daily MRHD, based on mg/m2).
In a peri- and post-natal toxicity study in rats, mebendazole did not adversely affect dams or their progeny at 20 mg/kg/day. At 40 mg/kg (1.9-fold the total daily MRHD, based on mg/m2), a reduction of the number of live pups was observed and there was no survival at weaning. No abnormalities were found on gross and radiographic examination of pups at birth.
8.2 Lactation
Risk Summary
Limited data from case reports demonstrate that a small amount of mebendazole is present in human milk following oral administration. There are no reports of effects on the breastfed infant, and the limited reports on the effects on milk production are inconsistent. The limited clinical data during lactation precludes a clear determination of the risk of EMVERM® to a breastfed infant; therefore, developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for EMVERM® and any potential adverse effects on the breastfed infant from EMVERM® or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of EMVERM® 100 mg chewable tablets has not been established in pediatric patients less than two years of age. Convulsions have been reported with mebendazole use in children less than one year of age [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
EMVERM® (mebendazole) is an orally administered, synthetic anthelmintic available as chewable tablets, each containing 100 mg of mebendazole, USP. Inactive ingredients are: microcrystalline cellulose, corn starch, anhydrous lactose NF, sodium starch glycolate, magnesium stearate, stearic acid, sodium lauryl sulfate, sodium saccharin, and FD&C Yellow #6.
Chemically, mebendazole is methyl 5-benzoylbenzimidazole-2-carbamate with a molecular formula of C16H13N3O3 and the following structural formula:
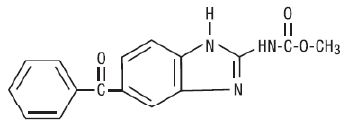
Mebendazole, USP is a white to slightly yellow powder with a molecular weight of 295.29. It is less than 0.05% soluble in water, dilute mineral acid solutions, alcohol, ether and chloroform, but is soluble in formic acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mebendazole, a benzimidazole, is an anthelmintic drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption
Following oral administration of mebendazole, the majority of the dose remains in the gastrointestinal tract where it exerts an anthelmintic effect locally. Following administration of 100 mg twice daily for three consecutive days, plasma concentrations of EMVERM® (mebendazole) and its primary metabolite, the 2-amine hydrolyzed metabolite, do not exceed 0.03 mcg/mL and 0.09 mcg/mL, respectively. Dosing with a high fat meal increases the bioavailability of mebendazole, although the overall effect of food on the amount of drug remaining in the gastrointestinal tract is not expected to be substantial.
Distribution
The plasma protein binding of mebendazole is 90 to 95%. The volume of distribution is 1 to 2 L/kg, indicating that absorbed mebendazole penetrates areas outside the vascular space.
Metabolism
Orally administered mebendazole is extensively metabolized primarily by the liver. Plasma concentrations of its major metabolites (hydrolyzed and reduced forms of mebendazole) are higher than those of mebendazole. All metabolites are devoid of anthelmintic activity. Impaired hepatic function, impaired metabolism, or impaired biliary elimination may lead to higher plasma concentrations of mebendazole.
Excretion
Mebendazole, the conjugated forms of mebendazole, and its metabolites likely undergo some degree of enterohepatic recirculation. The apparent elimination half-life after an oral dose ranges from 3 to 6 hours in most patients. Less than 2% of orally administered mebendazole is excreted in urine and the remainder in the feces as unchanged drug or its metabolites.
12.4 Microbiology
Mechanism of Action
Mebendazole interferes with cellular tubulin formation in the helminth and causes ultrastructural degenerative changes in its intestine. As a result, its glucose uptake and the digestive and reproductive functions are disrupted, leading to immobilization, inhibition of egg production and death of the helminth.
Antimicrobial Activity
Mebendazole is active against:
Ancylostoma duodenale
Ascaris lumbricoides
Enterobius vermicularis
Necator americanus
Trichuris trichiura
Resistance
There is a potential for development of resistance to mebendazole. The mechanism of resistance to mebendazole is likely due to changes of beta-tubulin protein, which reduces binding of mebendazole to beta-tubulin; however, the clinical significance of this is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In carcinogenicity tests of mebendazole in mice and rats, no carcinogenic effects were seen at doses as high as 40 mg/kg (one to two times the human dose, based on mg/m2) given daily over two years. No mutagenic activity was observed with mebendazole in a bacterial reverse gene mutation test. Mebendazole was mutagenic in the absence of S-9 when tested using a continuous (24 hour) treatment incubation period in the mouse lymphoma thymidine kinase assay. Mebendazole was aneugenic in vitro in mammalian somatic cells. In the in vivo mouse micronucleus assay, orally administered mebendazole induced an increased frequency of micronucleated polychromatic erythrocytes with evidence suggestive of aneugenicity. Doses up to 40 mg/kg in rats (2 times the total daily human dose, based on mg/m2), given to males for 60 days and to females for 14 days prior to gestation, had no effect upon fetuses and offspring.
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
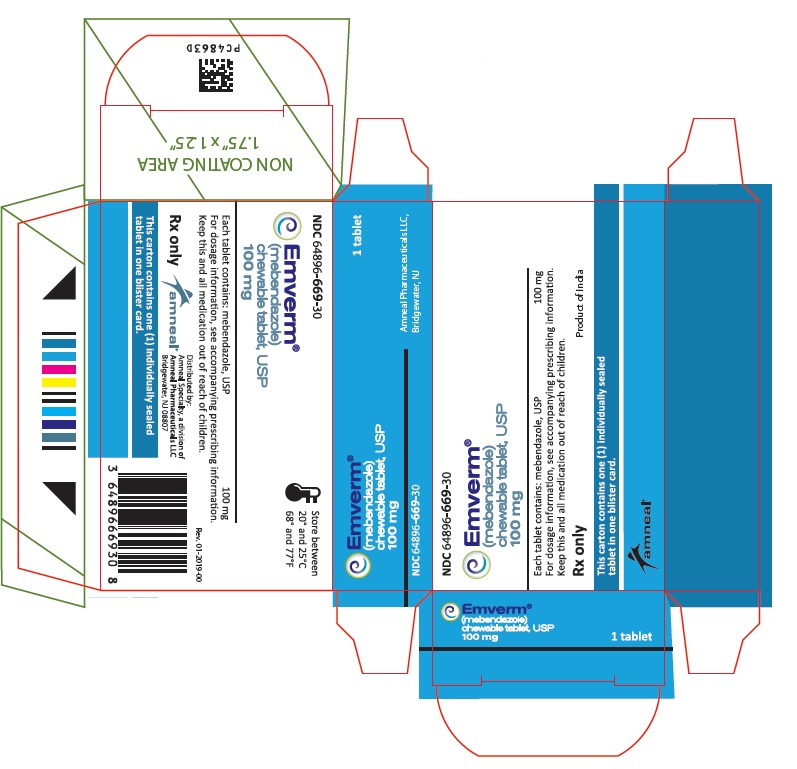
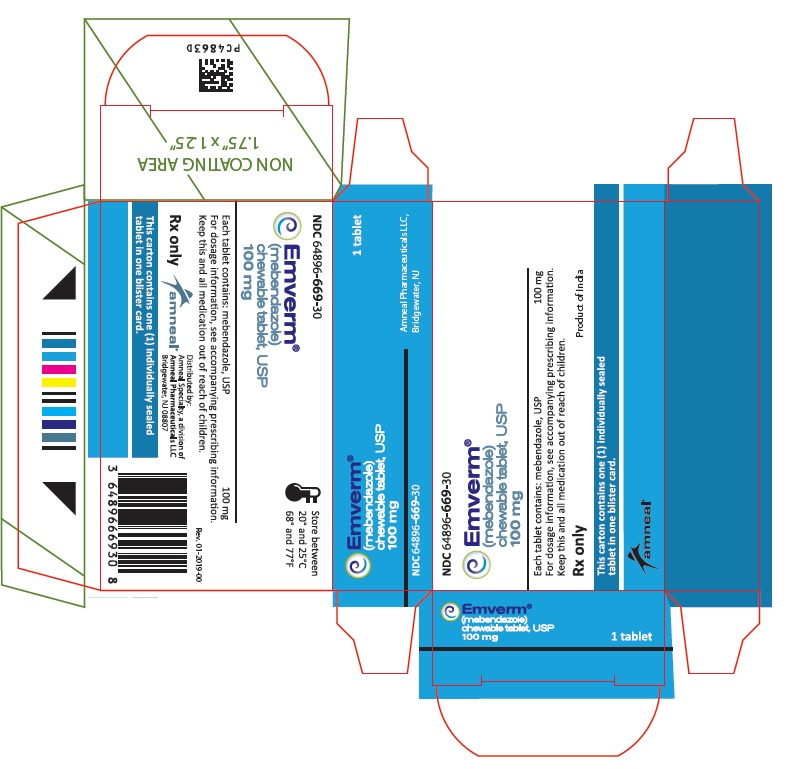
EMVERM® (mebendazole) Chewable Tablet, USP is available as a 100 mg, round, light peach-colored, unscored, debossed “ap” above “107” on one side and plain on the other side. They are supplied as follows:
Blister package of 1 tablet NDC 64896-669-30
Store at 68° to 77°F (20° to 25°C) [See USP Controlled Room Temperature].
- 17 PATIENT COUNSELING INFORMATION
- SPL UNCLASSIFIED SECTION
-
PATIENT INFORMATION
EMVERM® (EM-verm)
(mebendazole) Chewable Tablets USP, for oral useWhat is EMVERM?
Emverm is a prescription medicine used to treat adults and children 2 years of age and older with intestinal worm infections caused by pinworm, whipworm, roundworm, or hookworm.Who should not take EMVERM?
Do not take Emverm if you are allergic to mebendazole or any of the ingredients in Emverm. See the end of this leaflet for a complete list of ingredients in Emverm.Before you take EMVERM, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. It is not known if EMVERM will harm your unborn baby.
- are breastfeeding or plan to breastfeed. EMVERM can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take EMVERM. Do not breastfeed while taking EMVERM.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using EMVERM with certain other medicines can change the way these medicines act, causing serious side effects.
Know the medicines you take. Keep a list of them to show to your healthcare provider or pharmacist when you get a new medicine.How should I take EMVERM? -
Take EMVERM exactly as your healthcare provider tells you to take it.
-
Take EMVERM by mouth with or without food.
-
EMVERM tablets may be chewed, swallowed, or crushed and mixed with food.
-
If you take too much EMVERM, you might have symptoms that include stomach cramps, nausea, vomiting or diarrhea.
What should I avoid while taking EMVERM?
Do not take Emverm with metronidazole (a medicine used to treat bacterial and protozoan infections) as serious skin reactions called Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) can happen.What are the possible side effects of EMVERM?
EMVERM may cause serious side effects, including:-
Low white blood cell count (neutropenia). Neutropenia can cause you to get other infections. Your healthcare provider will check your blood count regularly during your treatment with EMVERM. Tell your healthcare provider right away if you have a fever or any signs of an infection while taking EMVERM
-
Severe skin reactions (Stevens-Johnson syndrome and toxic epidermal necrolysis). EMVERM may cause rare, but serious skin reactions when taken with metronidazole and other medicines that contain mebendazole. These severe allergic reactions may be life-threatening and need to be treated in a hospital. Call your healthcare provider right away or get emergency medical help if you have any allergic reactions or the following symptoms:
- severe skin blisters
- sores around the mouth, nose, eyes, vagina or penis (genitals)
- peeling skin
- swollen face, lips, mouth, tongue or throat
- itchy rash (hives)
The most common side effects of EMVERM include:
- loss of appetite (anorexia)
- stomach pain
- diarrhea
- passing gas
- nausea
- vomiting
- rash
Tell your healthcare provider if you have any side effect that bothers you or does not go away.
These are not all the possible side effects of EMVERM.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store EMVERM?
-
Store at room temperature between 68°F to 77°F (20°C to 25°C).
-
Safely throw away medicine that is out of date or no longer needed.
General information about the safe and effective use of EMVERM.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use EMVERM for a condition for which it was not prescribed. Do not give EMVERM to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about EMVERM that is written for health professionals.What are the ingredients in EMVERM?
Active ingredient: mebendazole, USP.
Inactive ingredients: microcrystalline cellulose, corn starch, anhydrous lactose NF, sodium starch glycolate, magnesium stearate, stearic acid, sodium lauryl sulfate, sodium saccharin, and FD&C Yellow #6.
Manufactured by:
Alcami
Wilmington, NC 28405
Distributed by:
Amneal Specialty, a division of
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
For more information, call Amneal Pharmaceuticals at 1-877-835-5472.This Patient Information has been approved by the U.S. Food and Drug Administration.
Rev. 08-2021-01
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EMVERM
mebendazole tablet, chewableProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:64896-669 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MEBENDAZOLE (UNII: 81G6I5V05I) (MEBENDAZOLE - UNII:81G6I5V05I) MEBENDAZOLE 100 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SACCHARIN SODIUM (UNII: SB8ZUX40TY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color orange (light peach) Score no score Shape ROUND Size 10mm Flavor Imprint Code ap;107 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64896-669-30 1 in 1 CARTON 02/15/2016 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073580 02/15/2016 Labeler - Amneal Pharmaceuticals LLC (123797875)