Label: 24HR HEALTH DAILY MEN- vitamin spray
- NHRIC Code(s): 80893-001-15
- Packager: USA Health
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated October 29, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Purpose
- Precaution
- Warning
- Safe Handling
- Dosage
- Supplement Facts
- Men's Daily
-
INGREDIENTS AND APPEARANCE
24HR HEALTH DAILY MEN
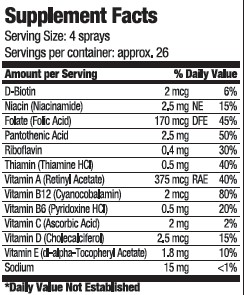
vitamin sprayProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:80893-001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 2 ug in 15 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2.5 mg in 15 mL FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 170 ug in 15 mL PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 2.5 mg in 15 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN .4 mg in 15 mL THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE .5 mg in 15 mL VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 375 ug in 15 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 2 ug in 15 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE .5 mg in 15 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 2 mg in 15 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 2.5 ug in 15 mL .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 1.8 mg in 15 mL SODIUM (UNII: 9NEZ333N27) (SODIUM - UNII:9NEZ333N27) SODIUM 15 mg in 15 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) SUCRALOSE (UNII: 96K6UQ3ZD4) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:80893-001-15 15 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 10/20/2020 Labeler - USA Health (117624854) Registrant - USA Health (117624854) Establishment Name Address ID/FEI Business Operations Streamline Manufacturing 098915617 manufacture