Label: IVERMECTIN cream
- NDC Code(s): 70771-1728-2, 70771-1728-3, 70771-1728-4
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated June 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED
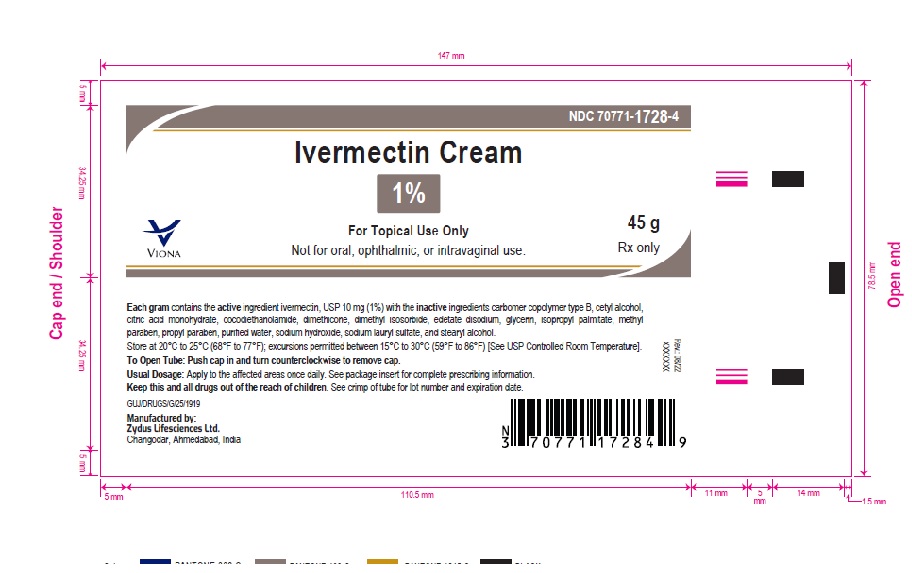
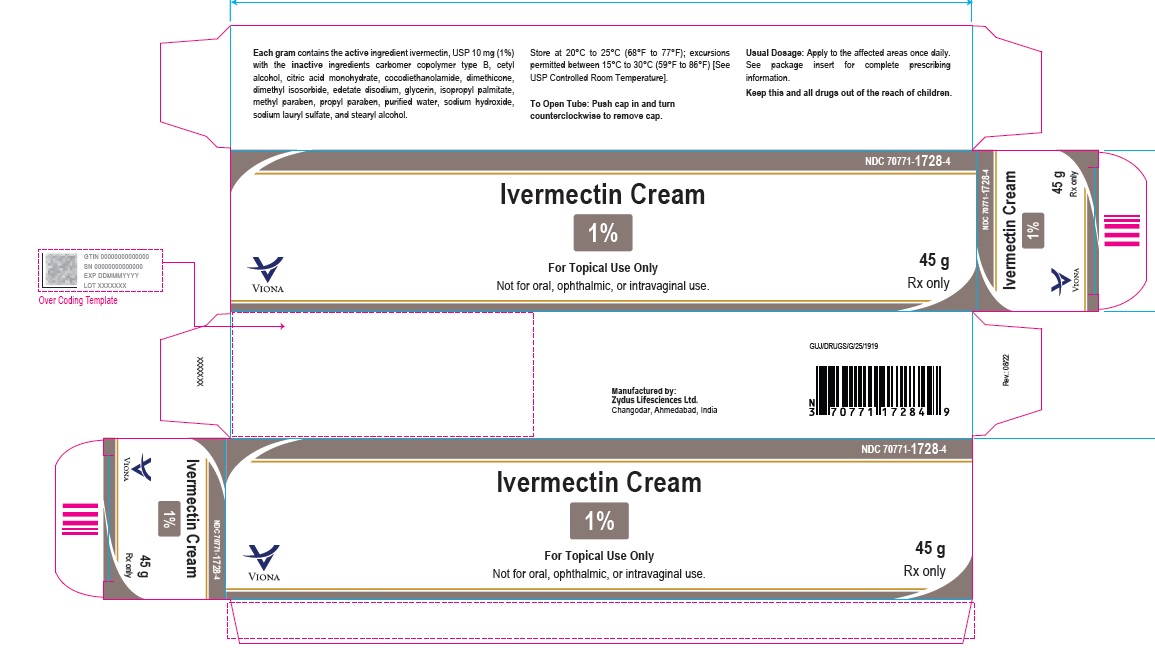
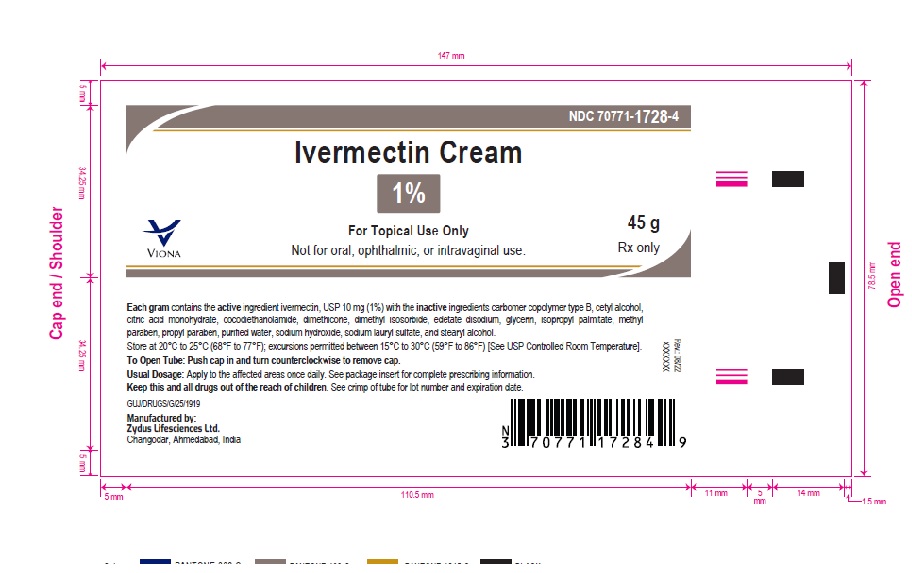
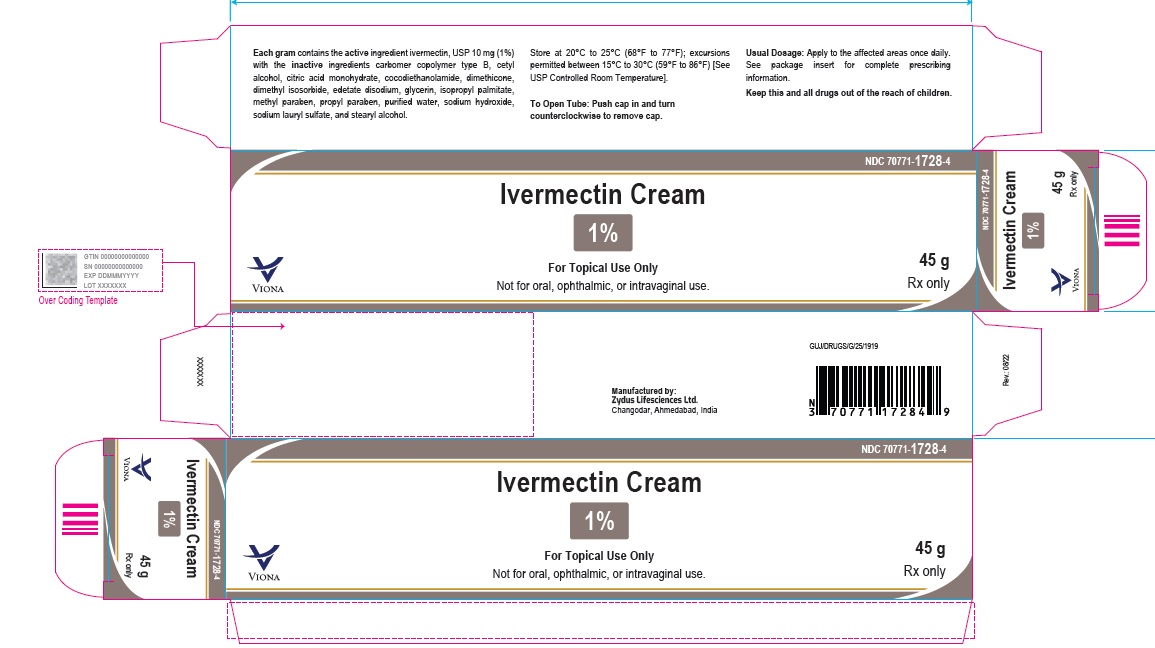
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IVERMECTIN
ivermectin creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1728 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IVERMECTIN (UNII: 8883YP2R6D) (IVERMECTIN - UNII:8883YP2R6D) IVERMECTIN 10 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER COPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 809Y72KV36) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO DIETHANOLAMIDE (UNII: 92005F972D) DIMETHICONE (UNII: 92RU3N3Y1O) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (white to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1728-2 1 in 1 CARTON 06/10/2023 1 30 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:70771-1728-4 1 in 1 CARTON 06/10/2023 2 45 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:70771-1728-3 1 in 1 CARTON 06/10/2023 3 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215210 06/10/2023 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 650650802 ANALYSIS(70771-1728) , MANUFACTURE(70771-1728)