Label: A AND D- dimethicone and zinc oxide cream
- NDC Code(s): 11523-1314-1, 11523-1314-2, 11523-1314-3
- Packager: Bayer HealthCare LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
- Inactive Ingredients
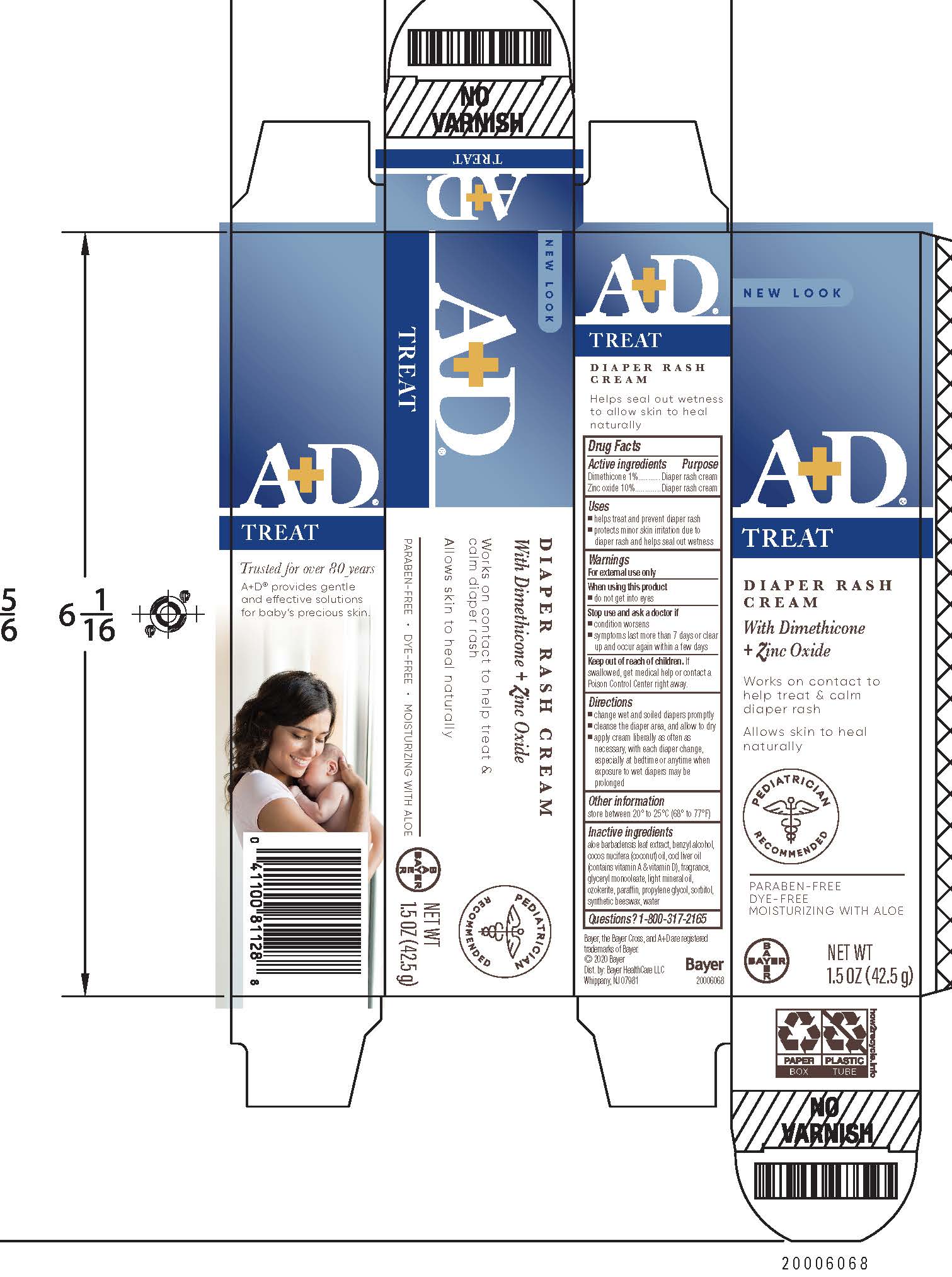
- PRINCIPAL DISPLAY PANEL - 113g Carton
-
INGREDIENTS AND APPEARANCE
A AND D
dimethicone and zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11523-1314 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 11.09 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 110.09 mg in 1 g Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) ALOE VERA FLOWER (UNII: 575DY8C1ER) BENZYL ALCOHOL (UNII: LKG8494WBH) COCONUT OIL (UNII: Q9L0O73W7L) COD LIVER OIL (UNII: BBL281NWFG) LIGHT MINERAL OIL (UNII: N6K5787QVP) PARAFFIN (UNII: I9O0E3H2ZE) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Product Characteristics Color white (White cream) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11523-1314-2 1 in 1 CARTON 12/25/2009 10/31/2019 1 113 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:11523-1314-3 1 in 1 CARTON 12/25/2009 2 85 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:11523-1314-1 1 in 1 CARTON 12/25/2009 05/11/2021 3 42.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/25/2009 Labeler - Bayer HealthCare LLC. (112117283)