Label: RABAVERT- rabies vaccine kit
- NDC Code(s): 50632-010-01, 50632-011-01, 50632-013-01
- Packager: Bavarian Nordic A/S
- Category: VACCINE LABEL
Drug Label Information
Updated April 17, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
RabAvert Rabies Vaccine is a sterile, freeze-dried vaccine obtained by growing the fixed-virus strain Flury Low Egg Passage (LEP) in primary cultures of chicken fibroblasts. The strain Flury LEP was obtained from American Type Culture Collection as the 59th egg passage. The growth medium for propagation of the virus is a synthetic cell culture medium with the addition of human albumin, polygeline (processed bovine gelatin), and antibiotics. The virus is inactivated with β-propiolactone and further processed by zonal centrifugation in a sucrose density gradient. The vaccine is lyophilized after addition of a stabilizer solution that consists of buffered polygeline and potassium glutamate. One dose of reconstituted vaccine contains ≤12 mg polygeline (processed bovine gelatin), ≤0.3 mg human serum albumin, 1 mg potassium glutamate, and 0.3 mg sodium EDTA. Small quantities of bovine serum are used in the cell culture process. Bovine components originate only from the United States, Australia, and New Zealand. Minimal amounts of chicken protein may be present in the final product; ovalbumin content is ≤3 ng/dose (1 mL), based on ELISA. Antibiotics (neomycin, chlortetracycline, amphotericin B) added during cell and virus propagation are largely removed during subsequent steps in the manufacturing process. In the final vaccine, neomycin is present at ≤10 mcg, chlortetracycline at ≤200 ng, and amphotericin B at ≤20 ng per dose. RabAvert is intended for intramuscular (IM) injection. The vaccine contains no preservative and should be used immediately after reconstitution with the supplied Sterile Diluent for RabAvert (Water for Injection). The potency of the final product is determined by the National Institutes of Health (NIH) mouse potency test using the United States (US) reference standard. The potency of 1 dose (1.0 mL) of RabAvert is at least 2.5 IU of rabies antigen. RabAvert is a white, freeze-dried vaccine for reconstitution with the diluent prior to use; the reconstituted vaccine is a clear to slightly opalescent, colorless to slightly pink suspension.
-
CLINICAL PHARMACOLOGY
Rabies in the United States: Over the last 100 years, the epidemiology of rabies in animals in the US has changed dramatically. More than 90% of all animal rabies cases reported annually to the Centers for Disease Control and Prevention (CDC) now occur in wildlife, whereas before 1960 the majority was in domestic animals. The principal rabies hosts today are wild terrestrial carnivores and bats. Annual human deaths have fallen from more than a hundred at the turn of the century to 1 to 2 per year despite major epizootics of animal rabies in several geographic areas. Within the US, only Hawaii has remained rabies free. Although rabies among humans is rare in the US, every year tens of thousands of people receive rabies vaccine for postexposure prophylaxis.
Rabies is a viral infection transmitted via the saliva of infected mammals. The virus enters the central nervous system of the host, causing an encephalomyelitis that is almost invariably fatal. The incubation period varies between 5 days and several years, but is usually between 20 and 60 days. Clinical rabies presents either in a furious or in a paralytic form. Clinical illness most often starts with prodromal complaints of malaise, anorexia, fatigue, headache, and fever followed by pain or paresthesia at the site of exposure. Anxiety, agitation, and irritability may be prominent during this period, followed by hyperactivity; disorientation; seizures; aerophobia and hydrophobia; hypersalivation; and eventually paralysis, coma, and death.
Modern day prophylaxis has proven nearly 100% successful; most human fatalities now occur in people who fail to seek medical treatment, usually because they do not recognize a risk in the animal contact leading to the infection. Inappropriate postexposure prophylaxis may also result in clinical rabies. Survival after clinical rabies is extremely rare, and is associated with severe brain damage and permanent disability.
RabAvert (in combination with passive immunization with Human Rabies Immune Globulin [HRIG] and local wound treatment) in postexposure treatment against rabies has been shown to protect patients of all age groups from rabies, when the vaccine was administered according to CDC’s Advisory Committee on Immunization Practices (ACIP) or World Health Organization (WHO) guidelines and as soon as possible after rabid animal contact. Anti-rabies antibody titers after immunization have been shown to reach levels well above the minimum antibody titer accepted as seroconversion (protective titer) within 14 days after initiating the postexposure treatment series. The minimum antibody titer accepted as seroconversion is a 1:5 titer (complete inhibition in the rapid fluorescent focus inhibition test [RFFIT] at 1:5 dilution) as specified by CDC1 or ≥0.5 IU/mL as specified by WHO.2,3
Clinical Studies:
Preexposure Vaccination: The immunogenicity of RabAvert was demonstrated in clinical trials conducted in different countries such as the US,4,5 the United Kingdom (UK),6 Croatia,7 and Thailand.8-10 When administered according to the recommended immunization schedule (Days 0, 7, and 21 or 0, 7, and 28), 100% of subjects attained a protective titer. In 2 studies carried out in the US in 101 subjects, antibody titers >0.5 IU/mL were obtained by Day 28 in all subjects. In studies carried out in Thailand in 22 subjects and in Croatia in 25 subjects, antibody titers of >0.5 IU/mL were obtained by Day 14 (injections on Days 0, 7, and 21) in all subjects.
The ability of RabAvert to boost previously immunized subjects was evaluated in 3 clinical trials. In the Thailand study, preexposure booster doses were administered to 10 individuals. Antibody titers of >0.5 IU/mL were present at baseline on Day 0 in all subjects.9 Titers after a booster dose were enhanced from geometric mean titers (GMTs) of 1.91 to 23.66 IU/mL on Day 30. In an additional booster study, individuals known to have been immunized with Human Diploid Cell Vaccine (HDCV) were boosted with RabAvert. In this study, a booster response was observed on Day 14 for all individuals (22/22).11 In a trial carried out in the US,4 an IM booster dose of RabAvert resulted in a significant increase in titers in all subjects (35/35), regardless of whether they had received RabAvert or HDCV as the primary vaccine.
Persistence of antibody after immunization with RabAvert was evaluated. In a trial performed in the UK, neutralizing antibody titers >0.5 IU/mL were present 2 years after immunization in all sera (6/6) tested.
Preexposure Vaccination in Children: Preexposure administration of RabAvert in 11 Thai children aged 2 years and older resulted in antibody levels higher than 0.5 IU/mL on Day 14 in all children.12
Postexposure Treatment: RabAvert, when used in the recommended postexposure WHO program of 5 to 6 IM injections of 1 mL (Days 0, 3, 7, 14, and 30 and optionally on Day 90) provided protective titers of neutralizing antibody (>0.5 IU/mL) in 158/160 patients8,9,13-16 within 14 days and in 215/216 patients by Days 28 to 38.
Of these, 203 were followed for at least 10 months. No case of rabies was observed.8,9,13-20 Some patients received HRIG, 20 to 30 IU/kg body weight, or Equine Rabies Immune Globulin (ERIG), 40 IU/kg body weight, at the time of the first dose. In most studies,8,9,13,17 the addition of either HRIG or ERIG caused a slight decrease in GMTs which was neither clinically relevant nor statistically significant. In one study,16 patients receiving HRIG had significantly lower (P<0.05) GMTs on Day 14; however, this was not clinically relevant. After Day 14 there was no statistical significance.
The results of several studies of normal volunteers receiving the postexposure WHO regimen, i.e., “simulated” postexposure, showed that with sampling by Days 28 to 30, 205/208 vaccinees had protective titers >0.5 IU/mL.
No postexposure vaccine failures have occurred in the US since cell culture vaccines have been routinely used.1 Failures have occurred abroad, almost always after deviation from the recommended postexposure treatment protocol.21-24 In 2 cases with bites to the face, treatment failed although no deviation from the recommended postexposure treatment protocol appeared to have occurred.25
Postexposure Treatment in Children: In a 10-year serosurveillance study, RabAvert was administered to 91 children aged 1 to 5 years and 436 children and adolescents aged 6 to 20 years.19 The vaccine was effective in both age groups. None of these patients developed rabies.
One newborn received RabAvert on an immunization schedule of Days 0, 3, 7, 14, and 30; the antibody concentration on Day 37 was 2.34 IU/mL. There were no clinically significant adverse events.26
-
INDICATIONS AND USAGE
RabAvert is indicated for preexposure vaccination, in both primary series and booster dose, and for postexposure prophylaxis against rabies in all age groups.
Usually an immunization series is initiated and completed with 1 vaccine product. No clinical studies have been conducted that document a change in efficacy or the frequency of adverse reactions when the series is completed with a second vaccine product. However, for booster immunization, RabAvert was shown to elicit protective antibody level responses in persons tested who received a primary series with HDCV.4,11
Preexposure Vaccination: See Table 1 and DOSAGE AND ADMINISTRATION.
Preexposure vaccination consists of 3 doses of RabAvert 1.0 mL given intramuscularly (deltoid region), 1 each on Days 0, 7, and 21 or 281 (see also Table 1 for criteria for preexposure vaccination).
Preexposure vaccination does not eliminate the need for additional therapy after a known rabies exposure (see DOSAGE AND ADMINISTRATION: Postexposure Prophylaxis of Previously Immunized Persons).
Preexposure vaccination should be offered to persons in high-risk groups, such as veterinarians, animal handlers, wildlife officers in areas where animal rabies is enzootic, certain laboratory workers, and persons spending time in foreign countries where rabies is endemic. Persons whose activities bring them into contact with potentially rabid dogs, cats, foxes, skunks, bats, or other species at risk of having rabies should also be considered for preexposure vaccination. International travelers might be candidates for preexposure vaccination if they are likely to come in contact with animals in areas where dog rabies is enzootic and immediate access to appropriate medical care, including biologics, might be limited.27,28
Preexposure vaccination is given for several reasons. First, it may provide protection to persons with inapparent exposure to rabies. Second, it may protect persons whose postexposure therapy might be expected to be delayed. Finally, although it does not eliminate the need for prompt therapy after a rabies exposure, it simplifies therapy by eliminating the need for globulin and decreasing the number of doses of vaccine needed. This is of particular importance for persons at high risk of being exposed in countries where the available rabies-immunizing products may carry a higher risk of adverse reactions.
In some instances, booster doses of vaccine should be administered to maintain a serum titer corresponding to at least complete neutralization at a 1:5 serum dilution by the RFFIT (Table 1); each booster immunization consists of a single dose. See CLINICAL PHARMACOLOGY. Serum antibody determinations to decide upon the need for a booster dose is suggested by ACIP and is considered cost effective.
Table 1. Rabies Preexposure Prophylaxis Guide – United States, 1999a a Adapted from the Recommendations of the Advisory Committee on Immunization Practices: Human Rabies Prevention – United States, 1999.1
b Judgment of relative risk and extra monitoring of vaccination status of laboratory workers is the responsibility of the laboratory supervisor.29
c Minimum acceptable antibody level is complete virus neutralization at a 1:5 serum dilution by rapid fluorescent focus inhibition test. A booster dose should be administered if the titer falls below this level.Risk Category and Nature of Risk
Typical Populations
Preexposure Prophylaxis Recommendations
Continuous. Virus present continuously, often in high concentrations. Specific exposures likely to go unrecognized. Bite, non-bite, or aerosol exposure.
Rabies research lab workers,b rabies biologics production workers.
Primary course. Serologic testing every 6 months; booster vaccination if antibody titer is below acceptable level.b
Frequent. Exposure usually episodic, with source recognized, but exposure might be unrecognized. Bite, non-bite, or aerosol exposure.
Rabies diagnostic lab workers,b spelunkers, veterinarians and staff, and animal-control and wildlife workers in rabies enzootic areas.
Primary course. Serologic testing every 2 years; booster vaccination if antibody titer is below acceptable level.c
Infrequent (greater than population-at-large). Exposure nearly always episodic with source recognized. Bite or non-bite exposure.
Veterinarians and animal-control and wildlife workers in areas with low rabies rates. Veterinary students. Travelers visiting areas where rabies is enzootic and immediate access to appropriate medical care including biologics is limited.
Primary course. No serologic testing or booster vaccination.c
Rare (population-at-large). Exposures always episodic with source recognized. Bite or non-bite exposure.
US population-at-large, including persons in rabies-epizootic areas.
No vaccination necessary.
Postexposure Treatment: See Table 2 and DOSAGE AND ADMINISTRATION.
The following recommendations are only a guide. In applying them, take into account the animal species involved, the circumstances of the bite or other exposure, the immunization status of the animal, and presence of rabies in the region (as outlined below). Local or state public health officials should be consulted if questions arise about the need for rabies prophylaxis.1
Table 2. Rabies Postexposure Prophylaxis Guide – United States, 1999a a Adapted from the Recommendations of the Advisory Committee on Immunization Practices: Human Rabies Prevention – United States, 1999.1
b During the 10-day observation period, begin postexposure prophylaxis at the first sign of rabies in a dog, cat, or ferret that has bitten someone. If the animal exhibits clinical signs of rabies, it should be euthanized immediately and tested.
c The animal should be euthanized and tested as soon as possible. Holding for observation is not recommended. Discontinue vaccine if immunofluorescence test results of the animal are negative.Animal Type
Evaluation and Disposition of Animal
Postexposure Prophylaxis Recommendations
Dogs, cats, and ferrets
Healthy and available for 10 days’ observation
Should not begin prophylaxis unless animal develops clinical signs of rabiesb
Rabid or suspected rabid
Immediately vaccinate
Unknown (e.g., escaped)
Consult public health officials
Skunks, raccoons, bats, foxes, and most other carnivores
Regarded as rabid unless animal proven negative by laboratory testsc
Consider immediate vaccination
Livestock, small rodents, lagomorphs (rabbits and hares), large rodents (woodchucks and beavers), and other mammals
Consider individually
Consult public health officials. Bites of squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice, other small rodents, rabbits, and hares almost never require antirabies postexposure prophylaxis.
In the US, the following factors should be considered before antirabies treatment is initiated.
Species of Biting Animal: Wild terrestrial animals (especially skunks, raccoons, foxes, and coyotes) and bats are the animals most commonly infected with rabies and are the most important potential source of infection for both humans and domestic animals. Unless a wild animal is tested and shown not to be rabid, postexposure prophylaxis should be initiated upon bite or non-bite exposure to the animals (see definition in “Type of Exposure” below). If treatment has been initiated and subsequent testing in a qualified laboratory shows the exposing animal is not rabid, postexposure prophylaxis can be discontinued.1
The likelihood of rabies in a domestic animal varies from region to region; hence, the need for postexposure prophylaxis also varies.1
Small rodents (such as squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, and mice) and lagomorphs (including rabbits and hares) are almost never found to be infected with rabies and have not been known to transmit rabies to humans in the US. Bites from large rodents such as woodchucks (including groundhogs) and beavers should be considered as possible rabies exposures, especially in regions where rabies is enzootic in raccoons.30 In all cases involving rodents, the state or local health department should be consulted before a decision is made to initiate antirabies postexposure prophylaxis.1
Circumstances of Biting Incident: An UNPROVOKED attack is more likely than a provoked attack to indicate the animal is rabid. Bites inflicted on a person attempting to feed or handle an apparently healthy animal should generally be regarded as PROVOKED. A currently vaccinated dog, cat, or ferret is unlikely to become infected with rabies.1
Type of Exposure: Rabies is transmitted by introducing the virus into open cuts or wounds in skin or via mucous membranes. The likelihood of rabies infection varies with the nature and extent of exposure. Two categories of exposure should be considered:
Bite: Any penetration of the skin by teeth. Bites to highly innervated areas such as the face and hands carry the highest risk, but the site of the bite should not influence the decision to begin treatment. Recent epidemiologic data suggest that even the very limited injury inflicted by a bat bite (compared with lesions caused by terrestrial carnivores) should prompt consideration of postexposure prophylaxis unless the bat is available for testing and is negative for evidence of rabies.1
Non-bite: The contamination of open wounds, abrasions, mucous membranes, or theoretically, scratches with saliva or other potentially infectious material (such as neural tissue) from a rabid animal constitutes a non-bite exposure. In all instances of potential human exposures involving bats, and the bat is not available for testing, postexposure prophylaxis might be appropriate even if a bite, scratch, or mucous membrane exposure is not apparent when there is reasonable probability that such exposure might have occurred. Postexposure prophylaxis can be considered for persons who were in the same room as the bat and who might be unaware that a bite or direct contact had occurred (e.g., a sleeping person awakens to find a bat in the room or an adult witnesses a bat in the room with a previously unattended child, mentally disabled person, or intoxicated person) and rabies cannot be ruled out by testing the bat. Other contact by itself, such as petting a rabid animal and contact with blood, urine, or feces (e.g., guano) of a rabid animal, does not constitute an exposure and is not an indication for prophylaxis. Because the rabies virus is inactivated by desiccation and ultraviolet irradiation, in general, if the material containing the virus is dry, the virus can be considered noninfectious. Two cases of rabies have been attributed to probable aerosol exposures in laboratories, and 2 cases of rabies in Texas could possibly have been due to airborne exposures in caves containing millions of bats.1
The only documented cases for rabies from human-to-human transmission occurred in 8 patients, including 2 in the US, who received corneas transplanted from persons who died of rabies undiagnosed at the time of death.1 Stringent guidelines for acceptance of donor corneas have been implemented to reduce this risk.
Bite and non-bite exposure from humans with rabies theoretically could transmit rabies, but no laboratory-diagnosed cases occurring under such situations have been documented. Each potential exposure to human rabies should be carefully evaluated to minimize unnecessary rabies prophylaxis.1
Postexposure Treatment Schedule: See also DOSAGE AND ADMINISTRATION.
The essential components of rabies postexposure prophylaxis are prompt local treatment of wounds and administration of both HRIG and vaccine.
A complete course of postexposure treatment for previously unvaccinated adults and children consists of a total of 5 doses of vaccine, each 1.0 mL: one IM injection (deltoid) on each of Days 0, 3, 7, 14, and 28. For previously immunized adults and children, a total of 2 doses of vaccine, each 1.0 mL: one IM injection (deltoid) on each of Days 0 and 3. No HRIG should be administered to previously vaccinated persons as it may blunt their rapid memory response to rabies antigen.
Local Treatment of Wounds: Immediate and thorough washing of all bite wounds and scratches with soap and water is an important measure for preventing rabies. In animal studies, thorough local wound cleansing alone has been shown to reduce markedly the likelihood of rabies. Whenever possible, bite injuries should not be sutured to avoid further and/or deeper contamination. Tetanus prophylaxis and measures to control bacterial infection should be given as indicated.1
Postexposure Prophylaxis of Rabies: The regimen for postexposure prophylaxis depends on whether or not the patient has been previously immunized against rabies (see below). For persons who have not previously been immunized against rabies, the schedule consists of an initial IM injection of HRIG exactly 20 IU/kg body weight in total. If anatomically feasible, the FULL DOSE of HRIG should be thoroughly infiltrated in the area around and into the wounds. Any remaining volume of HRIG should be injected intramuscularly at a site distant from rabies vaccine administration. HRIG should never be administered in the same syringe or in the same anatomical site as the rabies vaccine. HRIG is administered only once (for specific instructions for HRIG use, see the product package insert). The HRIG injection is followed by a series of 5 individual injections of RabAvert (1.0 mL each) given intramuscularly on Days 0, 3, 7, 14, and 28. Postexposure rabies prophylaxis should begin the same day exposure occurred or as soon after exposure as possible. The combined use of HRIG and RabAvert is recommended by the CDC for both bite and non-bite exposures, regardless of the interval between exposure and initiation of treatment.
In the event that HRIG is not readily available for the initiation of treatment, it can be given through the seventh day after administration of the first dose of vaccine. HRIG is not indicated beyond the seventh day because an antibody response to RabAvert is presumed to have begun by that time.1
The sooner treatment is begun after exposure, the better. However, there have been instances in which the decision to begin treatment was made as late as 6 months or longer after exposure due to delay in recognition that an exposure had occurred. Postexposure antirabies treatment should always include administration of both passive antibody (HRIG) and immunization, with the exception of persons who have previously received complete immunization regimens (preexposure or postexposure) with a cell culture vaccine, or persons who have been immunized with other types of vaccines and have had documented rabies antibody titers. Persons who have previously received rabies immunization should receive 2 IM doses of RabAvert: one on Day 0 and another on Day 3. They should not be given HRIG as this may blunt their rapid memory response to rabies antigen.
Postexposure Prophylaxis Outside the United States: If postexposure treatment is begun outside the US with regimens or biologics that are not used in the US, it may be prudent to provide additional treatment when the patient reaches the US. State or local health departments should be contacted for specific advice in such cases.1
-
CONTRAINDICATIONS
Preexposure Prophylaxis:
Hypersensitivity: History of anaphylaxis to the vaccine or any of the vaccine components constitutes a contraindication to preexposure vaccination with this vaccine.
Postexposure Prophylaxis: In view of the almost invariably fatal outcome of rabies, there is no contraindication to postexposure prophylaxis, including pregnancy.1
-
WARNINGS
Patients considered to be at risk of a severe hypersensitivity reaction to the vaccine or any of the vaccine components should receive an alternative rabies vaccine if a suitable product is available.
Anaphylaxis, meningitis; neuroparalytic events such as encephalitis, transient paralysis; Guillain-Barré Syndrome; myelitis; retrobulbar neuritis; and multiple sclerosis have been reported to be temporally associated with the use of RabAvert. See PRECAUTIONS and ADVERSE REACTIONS. A patient’s risk of developing rabies must be carefully considered, however, before deciding to discontinue immunization.
For intramuscular use only. For adults, the deltoid area is the preferred site of immunization; for small children and infants, administration into the anterolateral zone of the thigh is preferred. The use of the gluteal region should be avoided, since administration in this area may result in lower neutralizing antibody titers.1
Unintentional intravascular injection may result in systemic reactions, including shock.
Syncope (fainting) can occur in association with administration of injectable vaccines, including RabAvert. Syncope can be accompanied by transient neurological signs such as visual disturbance, paresthesia, and tonic-clonic limb movements. Procedures should be in place to avoid falling injury and to restore cerebral perfusion following syncope. See PRECAUTIONS and ADVERSE REACTIONS.
Development of active immunity after vaccination may be impaired in immune-compromised individuals. Please refer to PRECAUTIONS: Drug Interactions.
This product contains albumin, a derivative of human blood. It is present in RabAvert at concentrations of ≤0.3 mg/dose. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeld-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.
-
PRECAUTIONS
General: The healthcare provider should question the patient, parent, or guardian about (1) the current health status of the vaccinee and (2) reactions to a previous dose of RabAvert or a similar product. Preexposure vaccination should be postponed in the case of sick and convalescent persons and those considered to be in the incubation stage of an infectious disease. A separate, sterile syringe and needle should be used for each patient. Needles must not be recapped and should be properly disposed of. As with any rabies vaccine, vaccination with RabAvert may not protect 100% of susceptible individuals.
Hypersensitivity: RabAvert contains residues of egg and chicken proteins, such as ovalbumin. In instances where individuals have developed clinical symptoms of anaphylaxis such as generalized urticaria, upper airway (lip, tongue, throat, laryngeal, or epiglottal) edema, laryngeal spasm or bronchospasm, hypotension, or shock, following exposure to egg or chicken protein, the vaccine should only be administered by personnel with the capability and facilities to manage anaphylaxis post vaccination.
Since reconstituted RabAvert contains processed bovine gelatin and trace amounts of neomycin, chlortetracycline, and amphotericin B, the possibility of allergic reactions in individuals hypersensitive to these substances should be considered when administering the vaccine.
Epinephrine injection (1:1,000) must be immediately available should anaphylactic or other allergic reactions occur.
When a person with a history of hypersensitivity must be given RabAvert, antihistamines may be given; epinephrine (1:1,000), volume replacement, corticosteroids, and oxygen should be readily available to counteract anaphylactic reactions.
Drug Interactions: Radiation therapy, antimalarials, corticosteroids, other immunosuppressive agents, and immunosuppressive illnesses can interfere with the development of active immunity after vaccination and may diminish the protective efficacy of the vaccine. Preexposure vaccination should be administered to such persons with the awareness that the immune response may be inadequate. Immunosuppressive agents should not be administered during postexposure therapy unless essential for the treatment of other conditions. When rabies postexposure prophylaxis is administered to persons receiving corticosteroids or other immunosuppressive therapy, or who are immunosuppressed, it is important that a serum sample on Day 14 (the day of the fourth vaccination) be tested for rabies antibody to ensure that an acceptable antibody response has been induced.1
HRIG must not be administered at more than the recommended dose, since active immunization to the vaccine may be impaired.
No data are available regarding the concurrent administration of RabAvert with other vaccines.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term studies with RabAvert have not been conducted to assess the potential for carcinogenesis, mutagenesis, or impairment of fertility.
Use in Pregnancy: Animal reproductive studies have not been conducted with RabAvert. It is also not known whether RabAvert can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. RabAvert should be given to a pregnant woman only if clearly needed. The ACIP has issued recommendations for use of rabies vaccine in pregnant women.1
Use in Nursing Mothers: It is not known whether RabAvert is excreted in animal or human milk, but many drugs are excreted in human milk. Although there are no data, because of the potential consequences of inadequately treated rabies exposure, nursing is not considered a contraindication to postexposure prophylaxis. If the risk of exposure to rabies is substantial, preexposure vaccination might also be indicated during nursing.
Pediatric Use: Children and infants receive the same dose of 1 mL, given intramuscularly, as do adults.
Only limited data on the safety and efficacy of RabAvert in the pediatric age group are available. However, in 3 studies some preexposure and postexposure experience has been gained12,19,26 (see CLINICAL PHARMACOLOGY: Clinical Studies).
-
ADVERSE REACTIONS
In very rare cases, neurological and neuroparalytical events have been reported in temporal association with administration of RabAvert (see WARNINGS). These include cases of hypersensitivity (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
The most commonly occurring adverse reactions are injection site reactions, such as injection site erythema, induration, and pain; flu-like symptoms, such as asthenia, fatigue, fever, headache, myalgia, and malaise; arthralgia; dizziness; lymphadenopathy; nausea; and rash.
A patient’s risk of acquiring rabies must be carefully considered before deciding to discontinue vaccination. Advice and assistance on the management of serious adverse reactions for persons receiving rabies vaccines may be sought from the state health department or CDC (see CONTRAINDICATIONS).
Local reactions such as induration, swelling, and reddening have been reported more often than systemic reactions. In a comparative trial in normal volunteers, Dreesen et al..4 described their experience with RabAvert compared with an HDCV rabies vaccine. Nineteen subjects received RabAvert and 20 received HDCV. The most commonly reported adverse reaction was pain at the injection site, reported in 45% of the HDCV group and 34% of the group receiving RabAvert. Localized lymphadenopathy was reported in about 15% of each group. The most common systemic reactions were malaise (15% RabAvert vs. 25% HDCV), headache (10% RabAvert vs. 20% HDCV), and dizziness (15% RabAvert vs. 10% HDCV). In a recent study in the US5, 83 subjects received RabAvert and 82 received HDCV. Again, the most common adverse reaction was pain at the injection site in 80% in the HDCV group and 84% in the group receiving RabAvert. The most common systemic reactions were headache (52% RabAvert vs. 45% HDCV), myalgia (53% RabAvert vs. 38% HDCV), and malaise (20% RabAvert vs. 17% HDCV). None of the adverse events were serious; almost all adverse events were of mild or moderate intensity. Statistically significant differences between vaccination groups were not found. Both vaccines were generally well tolerated.
Uncommonly observed adverse events include temperatures above 38°C (100°F), swollen lymph nodes, pain in limbs, and gastrointestinal complaints. In rare cases, patients have experienced severe headache, fatigue, circulatory reactions, sweating, chills, monoarthritis, and allergic reactions; transient paresthesias and 1 case of suspected urticaria pigmentosa have also been reported.
Observed During Clinical Practice (See WARNINGS and PRECAUTIONS): The following adverse reactions have been identified during post approval use of RabAvert. Because these reactions are reported voluntarily from a population of uncertain size, estimates of frequency cannot be made. These events have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to RabAvert, or a combination of these factors:
Allergic: Anaphylaxis, Type III hypersensitivity-like reactions, bronchospasm, urticaria, pruritus, edema.
Central Nervous System: Neuroparalysis, encephalitis, meningitis, transient paralysis, Guillain-Barré Syndrome, myelitis, retrobulbar neuritis, multiple sclerosis, presyncope, syncope, vertigo, visual disturbance.
Cardiac: Palpitations, hot flush.
Local: Extensive limb swelling.
Skin and Subcutaneous Tissue Disorders: Angioedema.
The use of corticosteroids to treat life-threatening neuroparalytic reactions may inhibit the development of immunity to rabies (see PRECAUTIONS, Drug Interactions).
Once initiated, rabies prophylaxis should not be interrupted or discontinued because of local or mild systemic adverse reactions to rabies vaccine. Usually such reactions can be successfully managed with anti-inflammatory and antipyretic agents.
Reporting of Adverse Events: Adverse events should be reported by the healthcare provider or patient to the US Department of Health and Human Services (DHHS) Vaccine Adverse Event Reporting System (VAERS). Report forms and information about reporting requirements or completion of the form can be obtained from VAERS by calling the toll-free number 1-800-822-7967.1 In the US, such events can be reported to Bavarian Nordic: phone: 1-800-675-9596.
-
DOSAGE AND ADMINISTRATION
For intramuscular use only. The individual dose for adults, children, and infants is 1 mL.
In adults, administer vaccine by IM injection into the deltoid muscle. In small children and infants, administer vaccine into the anterolateral zone of the thigh. The gluteal area should be avoided for vaccine injections, since administration in this area may result in lower neutralizing antibody titers. Care should be taken to avoid injection into or near blood vessels and nerves. After aspiration, if blood or any suspicious discoloration appears in the syringe, do not inject but discard contents and repeat procedure using a new dose of vaccine at a different site.
Preexposure Dosage:
Primary Immunization: In the US, ACIP recommends 3 injections of 1 mL each: 1 injection on Day 0 and 1 on Day 7, and 1 either on Day 21 or 28 (for criteria for preexposure vaccination, see Table 1).
Booster Immunization: The individual booster dose is 1 mL, given intramuscularly.
Booster immunization is given to persons who have received previous rabies immunization and remain at increased risk of rabies exposure by reasons of occupation or avocation.
Persons who work with live rabies virus in research laboratories or vaccine production facilities (for continuous-risk category, see Table 1) should have a serum sample tested for rabies antibodies every 6 months. The minimum acceptable antibody level is complete virus neutralization at a 1:5 serum dilution by RFFIT. A booster dose should be administered if the titer falls below this level.
The frequent-risk category includes other laboratory workers such as those doing rabies diagnostic testing, spelunkers, veterinarians and staff, and animal-control and wildlife officers in areas where rabies is epizootic. Persons in the frequent-risk category should have a serum sample tested for rabies antibodies every 2 years and if the titer is less than complete neutralization at a 1:5 serum dilution by RFFIT should have a booster dose of vaccine. Alternatively, a booster can be administered in the absence of a titer determination.
The infrequent-risk category, including veterinarians, animal-control and wildlife officers working in areas of low rabies enzooticity (infrequent-exposure group), and international travelers to rabies enzootic areas, do not require routine preexposure booster doses of RabAvert after completion of a full primary preexposure vaccination scheme (Table 1).
Postexposure Dosage: Immunization should begin as soon as possible after exposure. A complete course of immunization consists of a total of 5 injections of 1 mL each: 1 injection on each of Days 0, 3, 7, 14, and 28 in conjunction with the administration of HRIG on Day 0. For children, see PRECAUTIONS: Pediatric Use.
Begin with the administration of HRIG. Give 20 IU/kg body weight.
This formula is applicable to all age groups, including infants and children. The recommended dosage of HRIG should not exceed 20 IU/kg body weight because it may otherwise interfere with active antibody production. Since vaccine-induced antibody appears within 1 week, HRIG is not indicated more than 7 days after initiating postexposure prophylaxis with RabAvert. If anatomically feasible, the FULL DOSE of HRIG should be thoroughly infiltrated in the area around and into the wounds. Any remaining volume of HRIG should be injected intramuscularly at a site distant from rabies vaccine administration. HRIG should never be administered in the same syringe or in the same anatomical site as the rabies vaccine.
Because the antibody response following the recommended immunization regimen with RabAvert has been satisfactory, routine post-immunization serologic testing is not recommended. Serologic testing is indicated in unusual circumstances, as when the patient is known to be immunosuppressed. Contact the appropriate state health department or CDC for recommendations.
Postexposure Prophylaxis of Previously Immunized Persons: When rabies exposure occurs in a previously vaccinated person, that person should receive 2 IM (deltoid) doses (1 mL each) of RabAvert: one immediately and one 3 days later. HRIG should not be given in these cases. Persons considered to have been immunized previously are those who received a complete preexposure vaccination or postexposure prophylaxis with RabAvert or other tissue culture vaccines or have been documented to have had a protective antibody response to another rabies vaccine. If the immune status of a previously vaccinated person is not known, full postexposure antirabies treatment (HRIG plus 5 doses of vaccine) is recommended. In such cases, if a protective titer can be demonstrated in a serum sample collected before vaccine is given, treatment can be discontinued after at least 2 doses of vaccine.
Instructions for Reconstituting RabAvert: Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exists, the vaccine should not be administered.
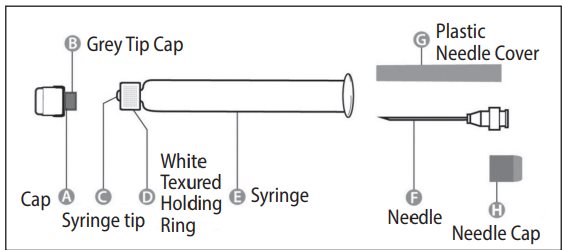
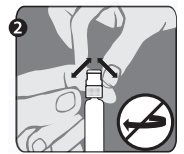
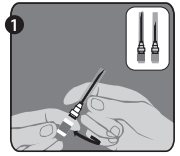
Needle application (these instructions apply to both the green and the orange needles):
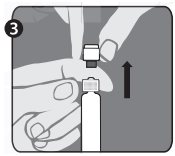
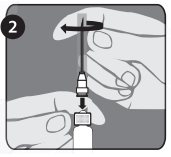
The package contains a vial of freeze-dried vaccine, a syringe containing 1 mL of sterile diluent, a sterile needle for reconstitution, and a sterile needle suitable for IM injection. The longer of the 2 needles supplied is the reconstitution needle. Affix the reconstitution needle to the syringe containing the Sterile Diluent for RabAvert. Insert the needle at a 45° angle and slowly inject the entire contents of the diluent (1 mL) into the vaccine vial. Mix gently to avoid foaming. The white, freeze-dried vaccine dissolves to give a clear to slightly opalescent, colorless to slightly pink suspension. Withdraw the total amount of dissolved vaccine into the syringe and replace the long needle with the smaller needle for IM injection. The reconstituted vaccine should be used immediately.
A separate sterile syringe and needle should be used for each patient. Needles must not be recapped and should be disposed of properly.
The lyophilization of the vaccine is performed under reduced pressure and the subsequent closure of the vials is done under vacuum. If there is no negative pressure in the vial, injection of Sterile Diluent for RabAvert would lead to an excess positive pressure in the vial. After reconstitution of the vaccine, it is recommended to unscrew the syringe from the needle to eliminate the negative pressure. After that, the vaccine can be easily withdrawn from the vial. It is not recommended to induce excess pressure, since over-pressurization may prevent withdrawing the proper amount of the vaccine.
-
HOW SUPPLIED
RabAvert product presentation is listed in Table 3.
Table 3. RabAvert Product Presentation Presentation
Carton NDC Number
Components
Single-dose kit
50632-010-01
- •
- 1 vial of freeze-dried vaccine containing a single dose [NDC 50632-013-01]
- •
- 1 disposable prefilled syringe of Sterile Diluent for reconstitution (1 mL) [NDC 50632-011-01]
- •
- 1 small needle for injection (25 gauge, 1 inch) and 1 long needle for reconstitution (21 gauge, 1 ½ inch)
RabAvert should be stored protected from light at 2°C to 8°C (36°F to 46°F). After reconstitution, the vaccine is to be used immediately. The vaccine may not be used after the expiration date given on package and container.
-
REFERENCES
1. CDC. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Human Rabies Prevention – United States, 1999. Morbidity and Mortality Weekly Report Recommendations and Report, January 8, 1999, Vol.48, RR-1; 1.1-21.
2. Smith JS, Yager, PA & Baer, GM. A rapid reproducible test for determining rabies neutralizing antibody. Bull WHO. 1973; 48: 535-541.
3. Eighth Report of the WHO Expert Committee on Rabies. WHO Technical Report Series, no. 824; 1992.
4. Dreesen DW, et al. Two-year comparative trial on the immunogenicity and adverse effects of purified chick embryo cell rabies vaccine for preexposure immunization. Vaccine. 1989; 7: 397-400.
5. Dreesen, DW. Investigation of antibody response to purified chick embryo cell tissue culture vaccine (PCECV) or human diploid cell culture vaccine (HDCV) in healthy volunteers. Study synopsis 7USA401RA, September 1996 – December 1996 (unpublished).
6. Nicholson KG, et al. Preexposure studies with purified chick embryo cell culture rabies vaccine and human diploid cell vaccine: serological and clinical responses in man. Vaccine. 1987; 5: 208-210.
7. Vodopija I, et al. An evaluation of second generation tissue culture rabies vaccines for use in man: a four-vaccine comparative immunogenicity study using a preexposure vaccination schedule and an abbreviated 2-1-1 postexposure schedule. Vaccine. 1986; 4: 245-248.
8. Wasi C, et al. Purified chick embryo cell rabies vaccine (letter). Lancet. 1986; 1: 40.
9. Wasi C. Rabies prophylaxis with purified chick embryo (PCEC) rabies vaccine. Protocol 8T--201RA, 1983 - 1984 (unpublished).
10. Wasi C. Personal communication to Behringwerke AG, 1990.
11. Bijok U, et al. Clinical trials in healthy volunteers with the new purified chick embryo cell rabies vaccine for man. J Commun Dis. 1984; 16: 61-69.
12. Lumbiganon P, et al. Preexposure vaccination with purified chick embryo cell rabies vaccines in children. Asian Pacific J Allergy Immunol. 1989; 7: 99-101.
13 Vodopija I. Post-exposure rabies prophylaxis with purified chick embryo cell (PCEC) rabies vaccine. Protocol 7YU-201RA, 1983-1985 (unpublished).
14. John J. Evaluation of purified chick embryo cell culture (PCEC) rabies vaccine, 1987 (unpublished).
15. Tanphaichitra D, Siristonpun Y. Study of the efficacy of a purified chick embryo cell vaccine in patients bitten by rabid animals. Intern Med. 1987; 3: 158-160.
16. Thongcharoen P, et al. Effectiveness of new economical schedule of rabies postexposure prophylaxis using purified chick embryo cell tissue culture rabies vaccine. Protocol 7T--301IP, 1993 (unpublished).
17. Ljubicic M, et al. Efficacy of PCEC vaccines in post-exposure rabies prophylaxis. In: Vodopija, Nicholson, Smerdel & Bijok (eds.): Improvements in rabies post-exposure treatment (Proceedings of a meeting in Dubrovnik, Yogoslavia). Zagreb Institute of Public Health 1985.17.
18. Madhusudana SN, Tripathi KK. Post exposure studies with human diploid cell rabies vaccine and purified chick embryo cell vaccine: Comparative Serological Responses in Man. Zbl Bakt 1989; 271: 345-350.
19. Sehgal S, et al. Ten year longitudinal study of efficacy and safety of purified chick embryo cell vaccine for pre- and postexposure prophylaxis of rabies in Indian population. J Commun Dis. 1995; 27: 36-43.
20. Sehgal S, et al. Clinical evaluation of purified chick embryo cell antirabies vaccine for postexposure treatment. J Commun Dis. 1988; 20: 293-300.
21. Fishbein DB, et al. Administration of human diploid-cell rabies vaccine in the gluteal area. N Engl J Med. 1988; 318: 124-125.
22. Shill M, et al. Fatal rabies encephalitis despite appropriate postexposure prophylaxis. A case report. N Engl J Med. 1987; 316: 1257-1258.
23. Wilde H, et al. Failure of rabies postexposure treatment in Thailand. Vaccine. 1989; 7: 49-52.
24. Kuwert EK, et al. postexposure use of human diploid cell culture rabies vaccine. Dev Biol Stand. 1977; 37: 273-286.
25. Hemachudha T, et al. Additional reports of failure to respond to treatment after rabies exposure in Thailand. Clin Infect Dis. 1999; 28: 143-144.
26. Lumbiganon P, Wasi C. Survival after rabies immunisation in newborn infant of affected mother. Lancet. 1990; 336: 319-320.
27. Centers for Disease Control and Prevention. Health Information for International Travel, 2003-2004 (The Yellow Book). Atlanta: US Department of Health and Human Services, Public Health Service, 2003. Internet version at: http://www.cdc.gov/travel/yb
28. World Health Organization. International Travel and Health, 2002. Geneva, Switzerland. Internet version at: http://www.who.int/ith
29. CDC and NIH. Biosafety in microbiological and biomedical laboratories. 3rd. ed. Washington, D.C. HHS Publication no. (CDC) 93-8395, Washington, DC: US Department of Health and Human Services, 1993.
30. Krebs JW, et al. Rabies surveillance in the United States in 2001. J Am Vet Med Assoc. 2002; 221: 1690-1701.
RabAvert is a trademark owned by Bavarian Nordic A/S.
Manufactured by:
Bavarian Nordic A/S, DK-2900 Hellerup, Denmark
US License No. 2096Distributed by:
Bavarian Nordic Inc.,
3025 Carrington Mill Boulevard, suite 100, NC 27560
©2019 Bavarian Nordic A/S.RAB:7PI
Rev. 10/2023 -
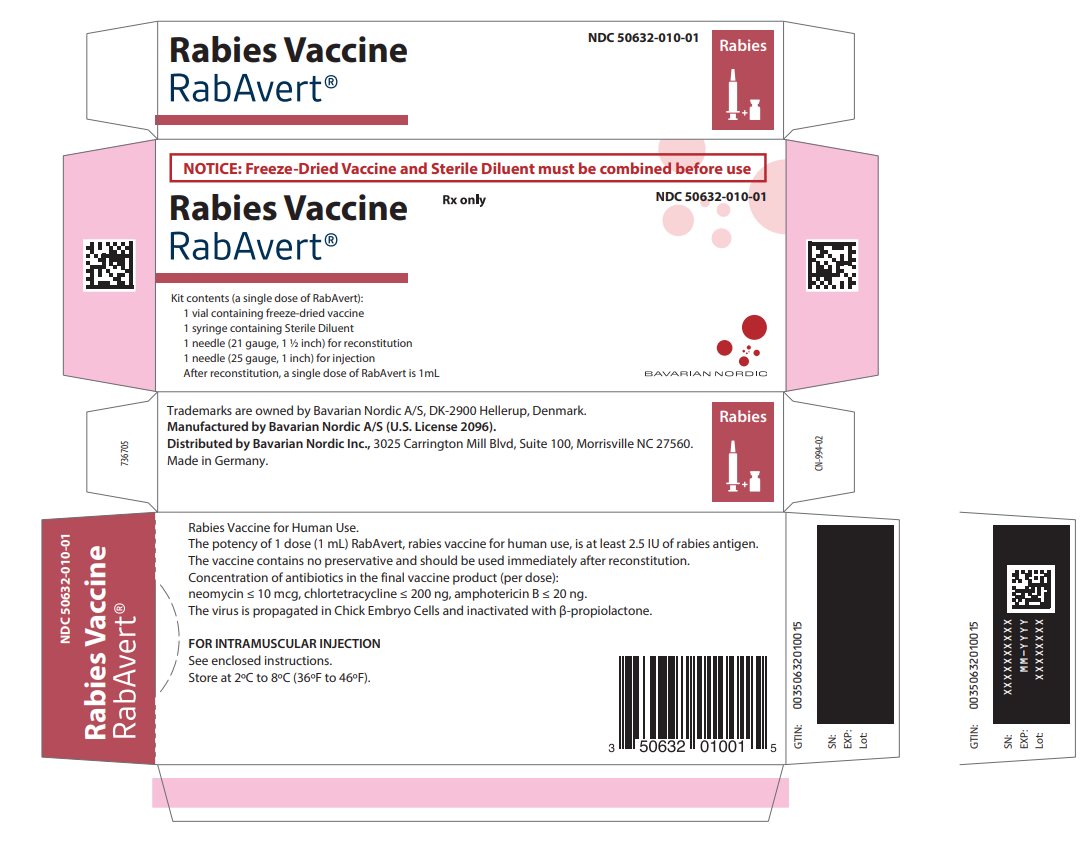
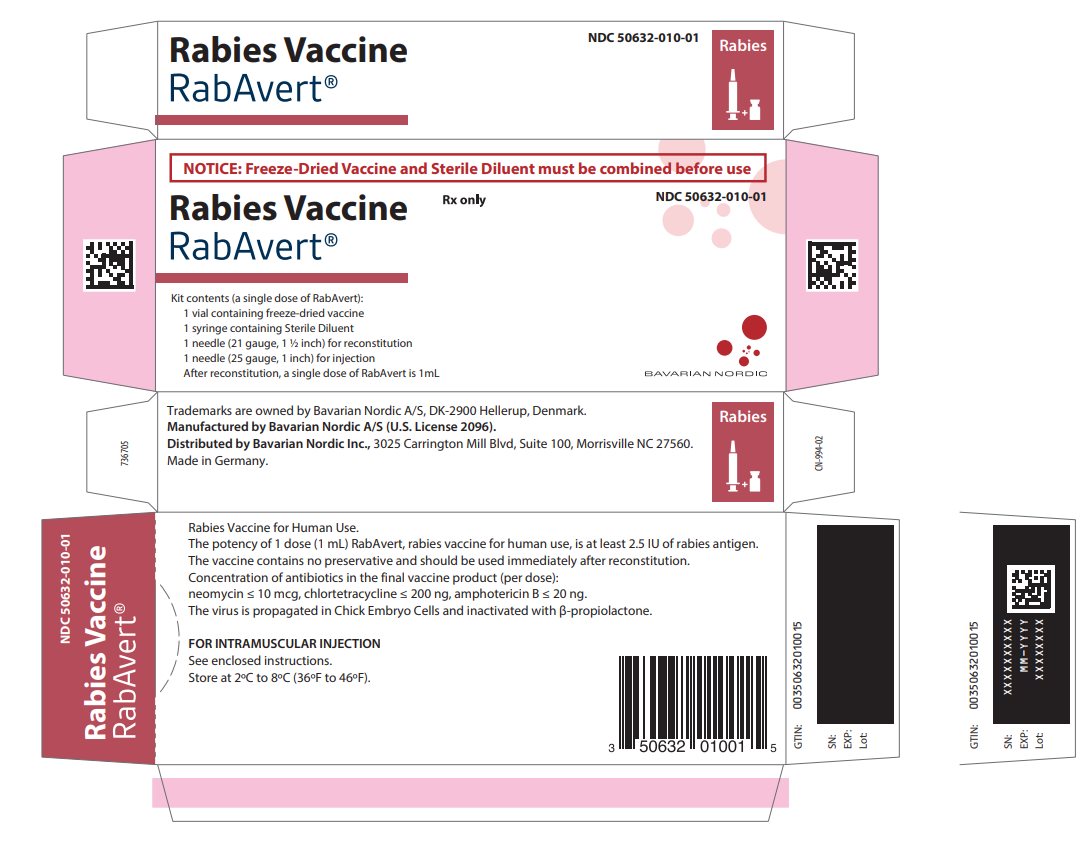
Principal Display Panel - Carton Label
NOTICE: Freeze-Dried Vaccine and Sterile Diluent must be combined before use
Rabies Vaccine
RabAvert®
Rx only
NDC 50632-010-01
Kit contents (a single dose of RabAvert):
1 vial containing freeze-dried vaccine
1 syringe containing Sterile Diluent
1 needle (21 gauge, 1 ½ inch) for reconstitution
1 needle (25 gauge, 1 inch) for injection
After reconstitution, a single dose of RabAvert is 1 mLBAVARIAN NORDIC
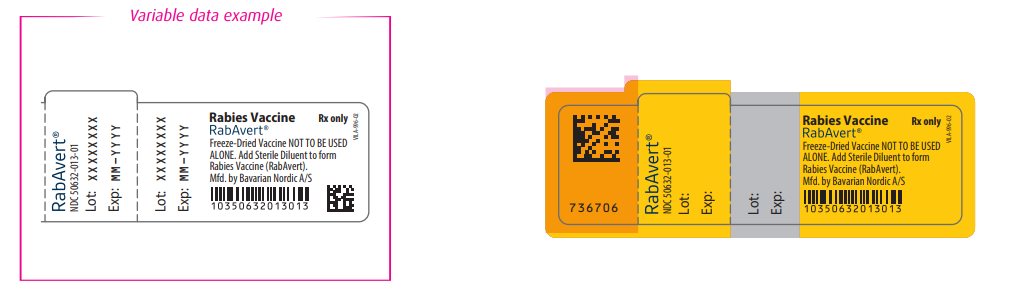
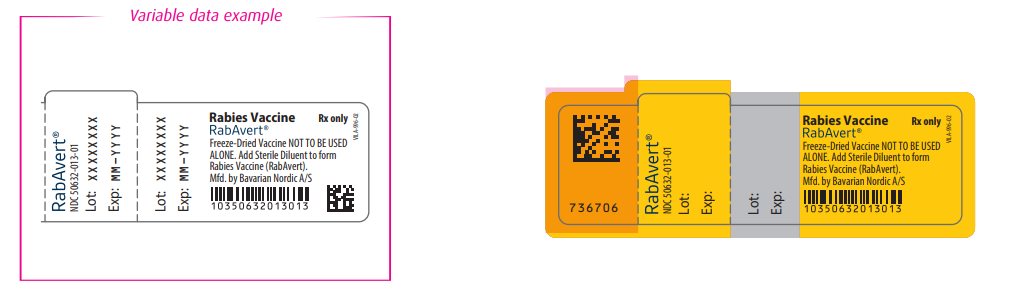
- Principal Display Panel - Vial Label
- Principal Display Panel - Diluent Label
-
INGREDIENTS AND APPEARANCE
RABAVERT
rabies vaccine kitProduct Information Product Type VACCINE Item Code (Source) NDC:50632-010 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50632-010-01 1 in 1 CARTON; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 1 mL Part 2 1 SYRINGE 1 mL Part 1 of 2 RABAVERT
rabies vaccine powder, for suspensionProduct Information Item Code (Source) NDC:50632-013 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RABIES VIRUS STRAIN FLURY LEP ANTIGEN (PROPIOLACTONE INACTIVATED) (UNII: FK894Q51YE) (RABIES VIRUS STRAIN FLURY LEP ANTIGEN (PROPIOLACTONE INACTIVATED) - UNII:FK894Q51YE) RABIES VIRUS STRAIN FLURY LEP ANTIGEN (PROPIOLACTONE INACTIVATED) 2.5 [iU] in 1 mL Inactive Ingredients Ingredient Name Strength Tromethamine (UNII: 023C2WHX2V) Sodium chloride (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM GLUCONATE (UNII: 12H3K5QKN9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50632-013-01 1 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103334 11/11/1997 Part 2 of 2 STERILE DILUENT
sterile diluent injectionProduct Information Item Code (Source) NDC:50632-011 Route of Administration INTRAMUSCULAR Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50632-011-01 1 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103334 12/19/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103334 11/11/1997 Labeler - Bavarian Nordic A/S (310209754)