Label: AI KUNGANG- calcium carbonate liquid
- NDC Code(s): 81768-0001-1
- Packager: TheJoenSpace Co,. LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
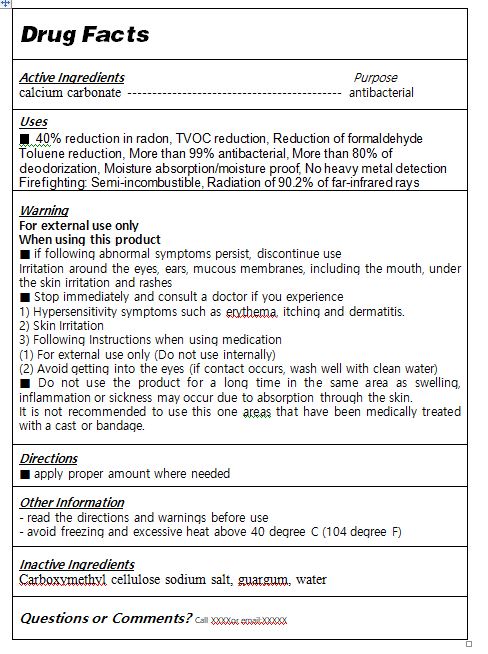
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- PURPOSE
-
WARNINGS
■ if following abnormal symptoms persist, discontinue use
Irritation around the eyes, ears, mucous membranes, including the mouth, under the skin irritation and rashes
■ Stop immediately and consult a doctor if you experience
1) Hypersensitivity symptoms such as erythema, itching and dermatitis.
2) Skin Irritation
3) Following Instructions when using medication
(1) For external use only (Do not use internally)
(2) Avoid getting into the eyes (if contact occurs, wash well with clean water)
■ Be careful not to inhale or use excessively for a long time (ingesting ethanol repeatedly causes irritation to mucous membranes and headaches or other symptoms may appear. When used repeatedly in the same area, skin irritation may occur.
■ Do not use the product for a long time in the same area as swelling, inflammation or sickness may occur due to absorption through the skin.
It is not recommended to use this one areas that have been medically treated with a cast or bandage.
■ Do not use in combination with soap or antibacterial cleansing agents.
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AI KUNGANG
calcium carbonate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81768-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 55 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81768-0001-1 2000 mL in 1 CAN; Type 0: Not a Combination Product 04/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/14/2021 Labeler - TheJoenSpace Co,. LTD (695644078) Registrant - TheJoenSpace Co,. LTD (695644078) Establishment Name Address ID/FEI Business Operations TheJoenSpace Co,. LTD 695644078 manufacture(81768-0001)